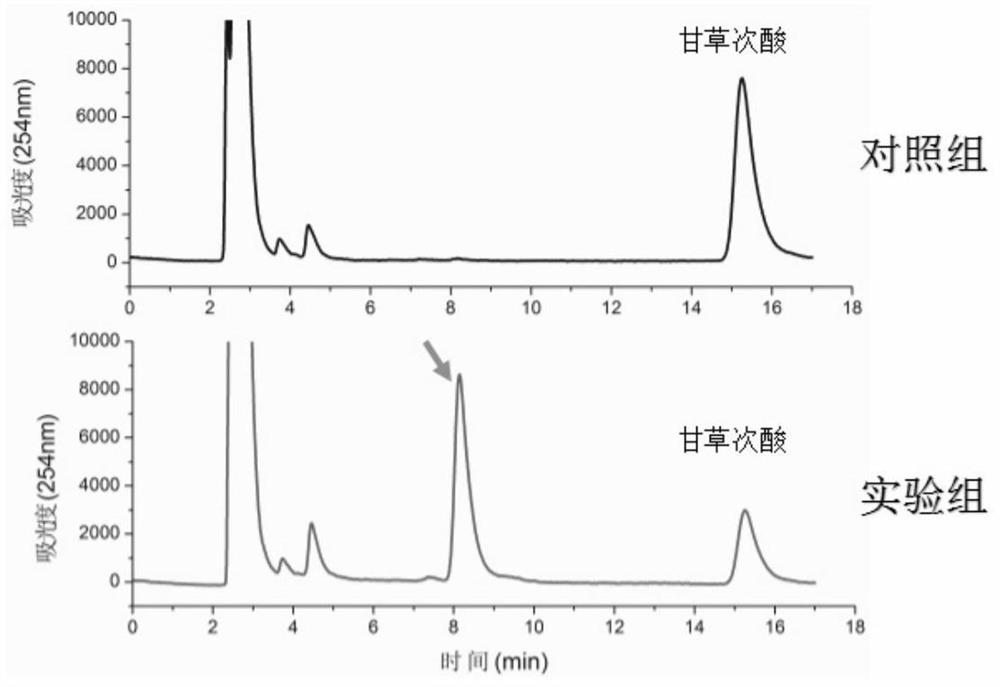
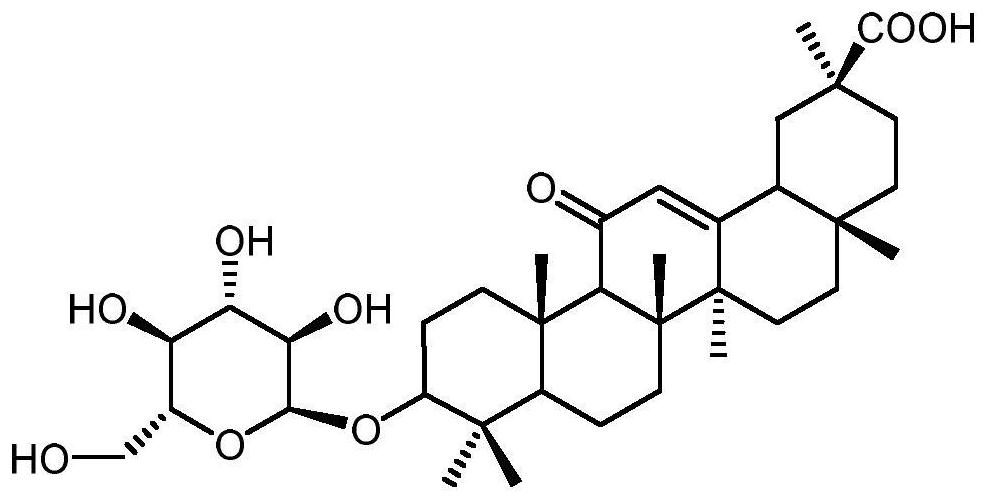
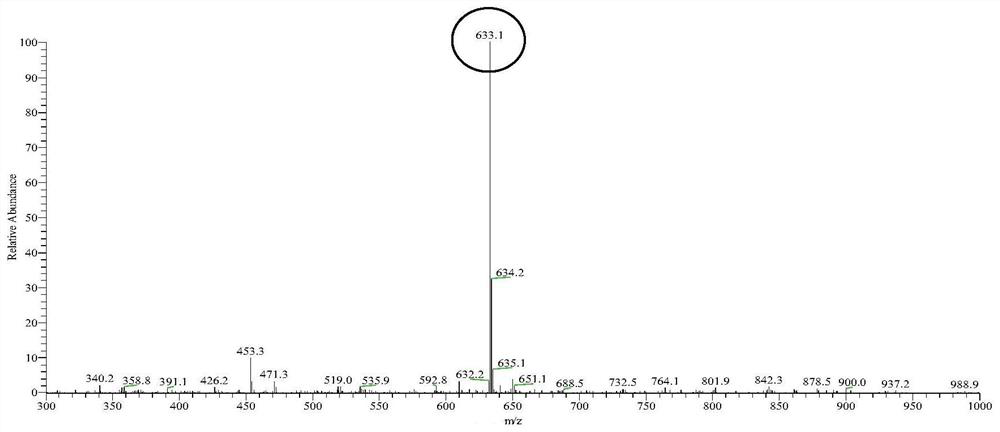
A method for enzymatically synthesizing 3-o-glucosylglycyrrhetinic acid
A glycyrrhetic acid and glucose-based technology, which is applied in the field of synthesizing 3-O-glucosylglycyrrhetic acid, can solve the problems of harsh reaction conditions, cumbersome reaction steps, reduced yield and the like, and achieves mild reaction conditions and high catalytic efficiency. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0036] Experimental Example 1: Synthesis of 3-O-glucosylglycyrrhetinic acid glycosyltransferase gene acquisition
[0037] A: The acquisition of glycosyltransferase Unigene14953 and Unigene20323 genes
[0038] According to the screening of the licorice transcriptome database, the glycosyltransferase genes Unigene14953 and Unigene20323 were obtained, and the primers were designed:
[0039] 53-F: 5'>ATGGACGTTG CTGAAGAACA GCC<3' (as shown in SEQ ID No.11);
[0040] 53-R: 5'>TTAGTTAAGC TGCTTAGAGT CTCTC<3' (as shown in SEQ ID No.12);
[0041] 23-F: 5'>ATGGTTTTCCAAAGCAAACCAACC<3' (as shown in SEQ ID No.13);
[0042]23-R: 5'>AGTTTCAACT TTACTACTTG ATTGT<3' (as shown in SEQ ID No.14);
[0043] Uralia licorice cDNA was used as a template, and ExTaq enzyme was used for PCR amplification to obtain the Unigene14953 gene of 1434bp fragment and the Unigene20323 gene of 1470bp fragment respectively, which were cloned into the pMD19-T vector as shown in SEQ ID No.1 and SEQ ID No.3 , the com...
Embodiment 2
[0053] Embodiment 2: Construct the Escherichia coli engineered bacterium containing glycosyltransferase
[0054] Construct Escherichia coli engineering bacteria E.coli BL21(DE3) / pET28a-UGT73C11 containing the glycosyltransferase UGT73C11 gene, chemically synthesize the glycosyltransferase UGT73C11 gene fragment (shown in SEQ ID No.8) as a template, and design upstream and downstream primers And add restriction sites EcoRI, SalI and protective bases:
[0055] E-C11-F: 5'>CCGGAATTCATGGTTTCTGAAATCACCCACAAAT<3' (as shown in SEQ ID No.19); with an EcoRI restriction site.
[0056] E-C11-R: 5'>ACGCGTCGACGTTGTTAGACTGAGCCAGCTGCATGAT<3' (as shown in SEQ ID No.20); with a SalI restriction site.
[0057] The PCR reaction system is: 1 μL of template, 2 μL of upstream and downstream primers, 25 μL of Primer star mix (Bao Biological Company), and less than 50 μL of double distilled water. PCR reaction conditions: pre-denaturation at 98°C for 1min, denaturation at 98°C for 10s, annealing at...
Embodiment 3
[0071] Embodiment 3: the fermentation of escherichia coli genetic engineering bacterium
[0072] (1) Pick and identify the correct Escherichia coli engineering bacteria and inoculate them in LB liquid medium (peptone 10g / L, yeast extract 5g / L, sodium chloride 10g / L) containing 100mg / L kanamycin for overnight culture , culture conditions 37°C, 170rpm.
[0073] (2) Inoculate 3 ml of the overnight cultured bacterial liquid into 300 ml LB liquid medium containing 100 mg / L kanamycin and cultivate for 3-5 hours until OD600=0.6, the culture condition is 37° C., 170 rpm.
[0074] (3) Add the inducer IPTG to make the final concentration 0.5mM / L, continue to cultivate, and the culture conditions are changed to 16°C, 170rpm, 8-10h.
[0075] (4) Centrifuge the obtained fermentation broth at room temperature at 8000 g for 10 min, and collect the bacteria.
[0076] (5) 25ml of 50mM PBS buffer solution with pH 7.0 was used to resuspend the bacteria, and the bacteria were lysed with a low-t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com