A foot-and-mouth disease vaccine mucosal immune enhancer, inactivated vaccine and preparation method thereof
A foot-and-mouth disease vaccine and Escherichia coli technology, applied in chemical instruments and methods, antiviral agents, pharmaceutical formulations, etc., can solve the problems of low antibody titer and non-detection, and achieve the effect of reducing labor costs and reducing the amount of virus carried
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1: Preparation of flagellin (F) and heat labile enterotoxin B subunit (LTB)
[0020] 1. Construction of recombinant plasmid
[0021] The complete amino acid sequence of the non-pathogenic E. coli flagellin (abbreviated as F) is shown in SEQ ID NO:1. The complete amino acid sequence of the heat labile enterotoxin B subunit (abbreviated as LTB) is shown in SEQ ID NO:2. The applicant used computer software to optimize the coding genes of F and LTB, and the optimized sequences are shown in SEQ ID NO: 3 and SEQ ID NO: 4, respectively. The codon optimized F and LTB coding genes were sent to GenScript for synthesis. The two synthetic sequences were cloned into the pCold I vector EcoR Ⅰ and Sal Between Ⅰ restriction sites, recombinant plasmids pCold I-F (with SEQ ID NO: 3 inserted) and pCold I-LTB (with SEQ ID NO: 4 inserted) were obtained.
[0022] 2. Construction and identification of recombinant bacteria
[0023] The obtained recombinant plasmids pCold I-F and pCold I-L...
Embodiment 2
[0034] Example 2 Preparation of Foot-and-Mouth Disease Vaccine Mucosal Immunity Enhancer and Control Immunity Enhancer
[0035] Recombinant proteins F and LTB were prepared according to the method in Example 1, FMDV inactivated virus solution (98 strains of porcine foot-and-mouth disease virus Burma, 4ug / ml) was provided by Lanzhou Veterinary Research Institute, and ISA206 was provided by Shanghai Seppic. PBS buffer (phosphate buffer, pH=7.4, 0.01mM) contains 0.27g / L potassium dihydrogen phosphate, 1.42g / L disodium hydrogen phosphate, 8g / L sodium chloride and 0.2g / L potassium chloride The aqueous solution was purchased from Nanjing Research Aid Biotechnology Co., Ltd.
[0036] 1. Foot-and-mouth disease vaccine mucosal immune enhancer and vaccine prepared by using the immune enhancer
[0037] (1) Preparation of mucosal immune enhancers A, B and C
[0038] Preparation of the recombinant protein F mother solution: The recombinant protein F purified in Example 1 was prepared into a solut...
Embodiment 3
[0050] Example 3: Immunity effects of foot-and-mouth disease vaccines A, B and C on pigs
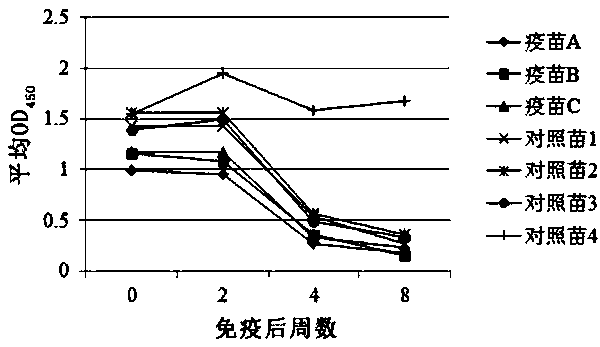
[0051] Immunization method: 35 healthy FMDV-negative piglets aged 60 days were randomly divided into 7 groups with 5 pigs in each group. A group of piglets were immunized with foot-and-mouth disease vaccine A, foot-and-mouth disease vaccine B, foot-and-mouth disease vaccine C, control vaccine 1, control vaccine 2, control vaccine 3, and control vaccine 4, each at a dose of 2 ml / head. 14 days, 28 days and 56 days after immunization, blood was collected to determine the level of IgG antibodies, and nasal swabs were collected to detect the level of IgA antibodies.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com