A kind of cabazitaxel phospholipid composition and its preparation method and application
A technology of cabazitaxel and its composition, which is applied in the field of pharmaceutical preparations, can solve the problems of affecting the curative effect, weakening the advantages of liposome preparations, liposomes are easy to aggregate, etc., to solve the problem of large and uneven particle size, and increase the stability in vivo and in vitro properties, and the effect of improving chemical stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1 Preparation of lyophilized powder of cabazitaxel phospholipid composition
[0039] Weigh 60 mg of cabazitaxel and 300 mg of DSPG and add it into 10 mL of chloroform:methanol (9:1, v / v) and stir at 60°C for 0.5 h until the solution is clear and transparent, then rotary evaporate to obtain the cabazitaxel lipoplex.
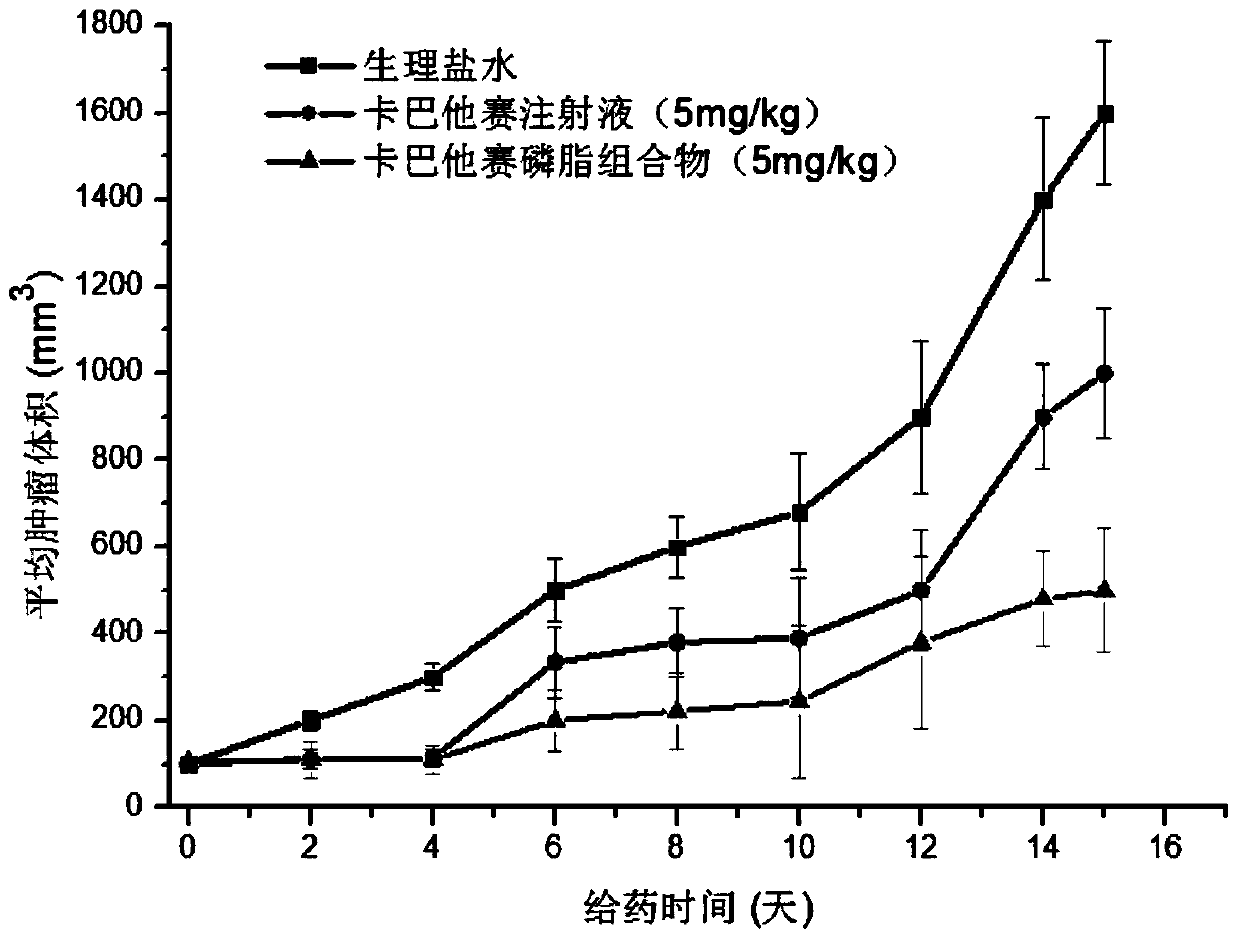
[0040] Take the above-mentioned cabazitaxel lipid complex, 1200mg HSPC, and 120mg cholesterol, dissolve them in 40mL chloroform:methanol (9:1, v / v) solution, and spray dry (inlet temperature: 45°C) to obtain white particles, add 30mL containing The aqueous solution of 10wt% sucrose was hydrated, and then homogenized under high pressure 5 times under the pressure of 20000psi to obtain the cabazitaxel phospholipid composition with light blue opalescence. °C for 5 h, primary drying: -30 °C for 10 h, secondary drying: at 4 °C for 10 h) to obtain the lyophilized powder of cabazitaxel phospholipid composition. After cabazitaxel phospholipid compositio...
Embodiment 2
[0041] The preparation of embodiment 2 cabazitaxel phospholipid composition injection
[0042] Weigh 60 mg of cabazitaxel and 600 mg of DMPG and add 20 mL of chloroform:methanol (1:1, v / v) at 40°C and stir for 1 hour until the solution is clear and transparent, then spray dry (inlet temperature: 55°C) to obtain cabazitaxel lipid complex thing.
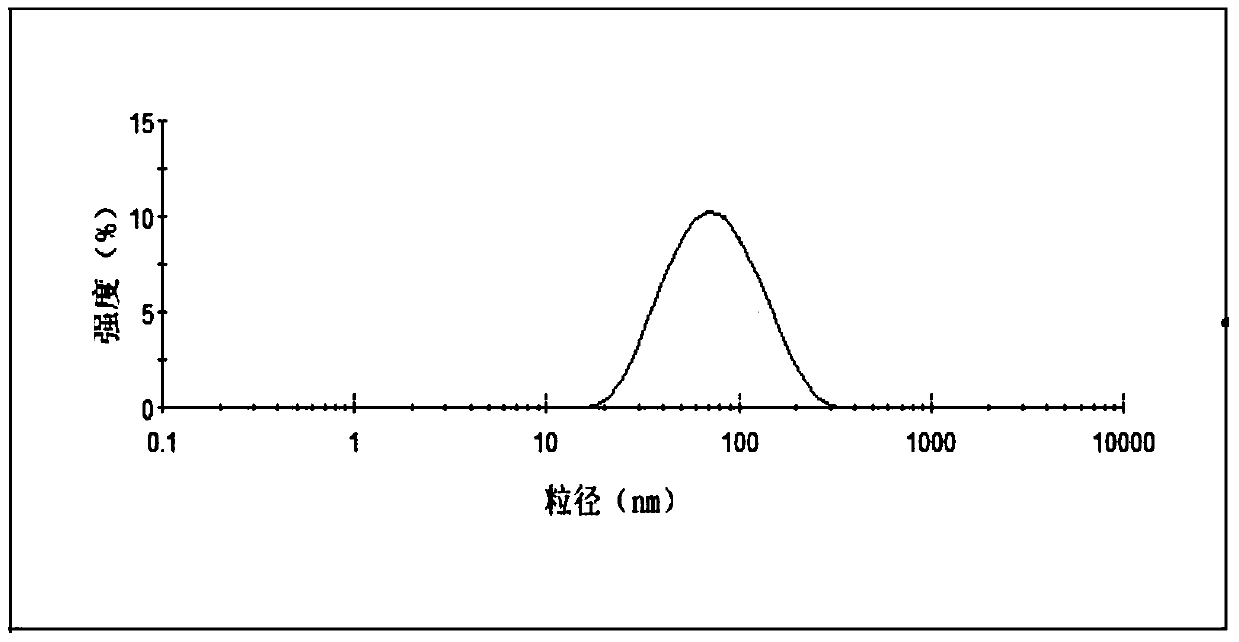
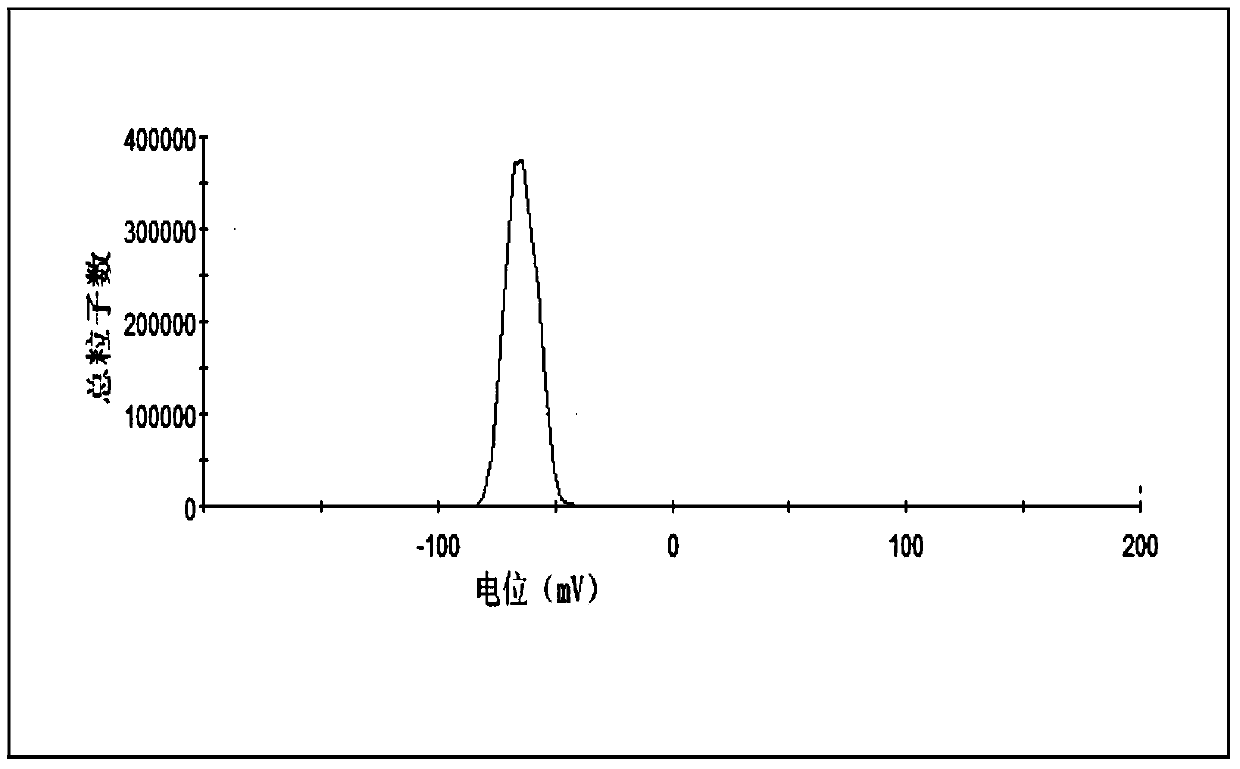
[0043] Take the above-mentioned cabazitaxel lipid complex, 1200mg soybean lecithin, and 200mg cholesterol in a 250mL round-bottomed flask, dissolve in 40mL chloroform:methanol (1:1, v / v) solution, and dry under reduced pressure at 45°C to remove organic matter. Solvent, form a lipid film on the wall of the round bottom flask, add 30mL of pure water to hydrate, and squeeze out 4 times through 0.2μm and 0.1μm microporous membrane filters in turn to obtain cabazitaxel phospholipid composition injection. The particle size and encapsulation efficiency of the taxel phospholipid composition are 80.5nm and 86.7% respectively.
Embodiment 3
[0044] Embodiment 3 Preparation of lyophilized powder of cabazitaxel phospholipid composition
[0045] Weigh 60 mg of cabazitaxel and 1200 mg of DPPG, add 20 mL of dichloromethane:methanol (1:1, v / v) and stir at 60°C for 1 hour until the solution is clear and transparent, then spray dry (inlet temperature: 55°C) to obtain cabazitaxel lipid substance complex.
[0046] Take the above-mentioned cabazitaxel lipid complex, 1800mg lecithin, and 200mg cholesterol in a 250mL round bottom flask, dissolve it with 40mL dichloromethane:methanol (1:1, v / v) solution, and dry it under reduced pressure at 45°C Remove the organic solvent, form a lipid film on the wall of the round bottom flask, add 30mL aqueous solution containing 10wt% sucrose and 5wt% lactose for hydration, and homogenize under high pressure for 4 times under the pressure of 20000psi, divide the resulting suspension into vials, Freeze-dry to obtain the freeze-dried powder of the cabazitaxel phospholipid composition. After ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com