Liposome of astragaloside IV and its medicinal preparation
A technology of astragaloside IV and astragaloside IV, which is applied in the field of liposome pharmaceutical preparations, can solve problems such as aggravating the burden on the heart and medical care of patients, increasing the burden on the heart of patients with heart failure, allergic reactions, pyrogen reactions, etc. Bioavailability, low toxicity, enhanced efficacy
Inactive Publication Date: 2010-12-01
TIANJIN INSTITUTE OF PHARMA RESEARCH
View PDF2 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
These problems include: 1. In order to prepare injections, the dissolution of astragaloside IV must be solved, so harmful cosolvents and surfactants are used for injection. 2. Patients with chronic heart failure have a long course of disease, and most of them do not need hospitalization. Long-term infusion will increase the patient's psychological burden and medical burden. 3. Intravenous infusion of drugs generally has a large input volume, which will increase the heart burden of heart failure patients and aggravate the degree of heart failure. There is a sense of fear in the pain of injection. If the medication cycle is long, this problem will become more prominent. 5. There are unsafe factors in the injection process, such as allergic reactions, pyrogen reactions, etc.
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Login to View More
Abstract
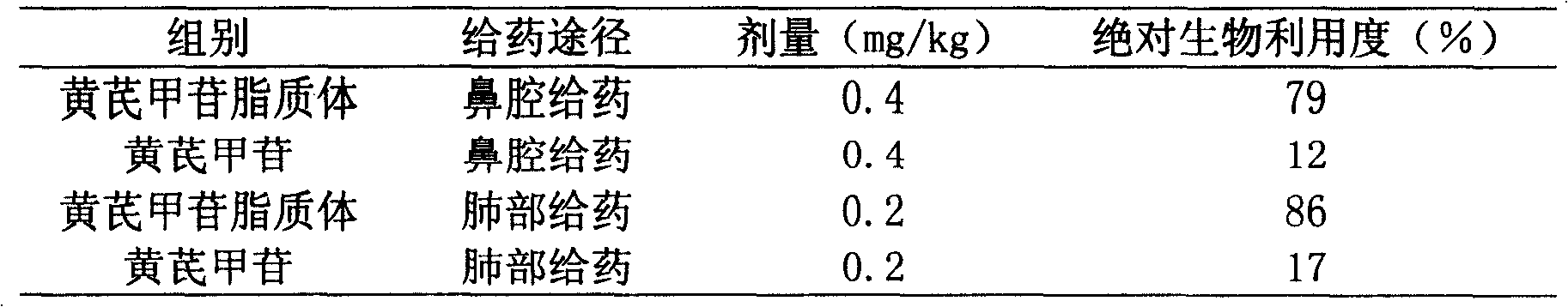
The invention relates to a astragaloside liposome and method for preparing same, wherein the liposome is prepared from astragaloside, phosphatide and lipophilic additives, the weight ratio of phosphatide and astragaloside is 0.5:1-100:1, the weight ratio of phosphatide and lipophilic additives is 1:1-50:1. The liposome can be used for preparing powdered inhalant, aerosols, sprays and oral mucosa doses.
Description
Astragaloside IV liposome and its pharmaceutical preparations technical field The invention belongs to the technical field of medicine and relates to a liposome pharmaceutical preparation, more specifically, an astragaloside IV liposome and a pharmaceutical preparation containing astragaloside IV liposome. Background technique Astragalus is one of the most commonly used traditional Chinese medicines with a long history and wide clinical application. Traditional Chinese medicine believes that astragalus is warm in nature and sweet in taste, and has the functions of invigorating qi and raising yang, solidifying the surface and antiperspirant, detoxifying and promoting muscle growth, reducing swelling and diuresis. Astragaloside IV is a monomeric compound extracted from the traditional Chinese medicine Astragalus membranaceus. In recent years, a large number of studies have shown that astragaloside IV can be used in the prevention and treatment of cardiovascular system diseas...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Patents(China)
IPC IPC(8): A61K31/7048A61K36/481A61K9/127A61K9/14A61K9/12A61K9/72A61P9/04
Inventor 王亚静李章才
Owner TIANJIN INSTITUTE OF PHARMA RESEARCH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com