Preparation method of flame retardant for inflammable hazardous chemical plastic products
A technology for flame retardants and hazardous chemicals, applied in the field of chemical preparation preparation, can solve the problem of easily turning yellow, orange or even purple in severe cases, product strength, hardness, elasticity, and active ingredient content of flame retardants. Advanced problems, to achieve the effect of strong flame retardancy, easy rubber mixing, and good compatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] A method for preparing a plastic flame retardant for flammable hazardous chemicals based on (2,4-dibromophenyl) phosphate triester, comprising the following process steps:
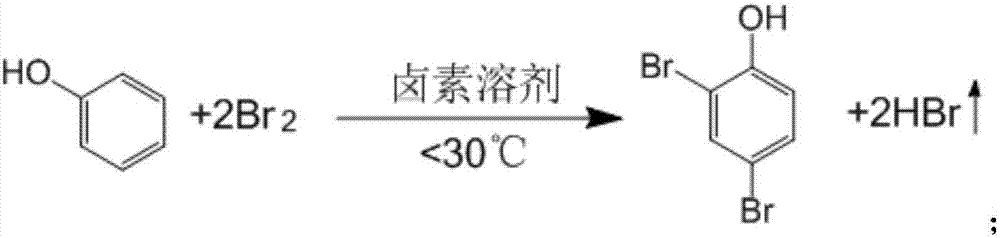
[0029] 1. Synthesis of 2,4-dibromophenol
[0030] Required equipment: clean four-necked glass reaction bottle, return pipe and matching external vacuum exhaust gas absorption bottle.
[0031] 1. Add 28.2g (0.3 mole) of dry industrial phenol and 300ml of chloroform to the four-necked bottle successively, then stir to dissolve, and control the temperature below 30°C;
[0032] 2. Slowly add 96g (0.6 mole) of dried industrial bromine dropwise to the reaction bottle, control the feeding speed for about 2 hours, control the reaction temperature not to exceed 30°C, after the dropwise addition is completed, then continue the reflux reaction in a boiling state 1.5h to obtain 2,4-dibromophenol solution;
[0033] 3. The reaction tail gas is absorbed by water to produce hydrobromic acid, which is sold as a by...
Embodiment 2
[0045] A method for preparing a plastic flame retardant for flammable hazardous chemicals based on (2,4-dibromophenyl) phosphate triester, comprising the following process steps:
[0046] 1. Synthesis of 2,4-dibromophenol
[0047] 1. In a 2000ml four-necked bottle, add 112.8g (1.2 moles) of dry industrial phenol and 1200ml chloroform to dissolve it under stirring;
[0048] 2. Control the reaction temperature below 30°C, slowly add 384g (2.4 moles) of industrial bromine dropwise under stirring, control the addition in 1 hour, control the reaction temperature not to exceed 30°C, and continue the reflux reaction for 1.5h to obtain 2,4― Dibromophenol solution;
[0049] 3. The reaction tail gas is made into hydrobromic acid as a by-product through the water absorption device.
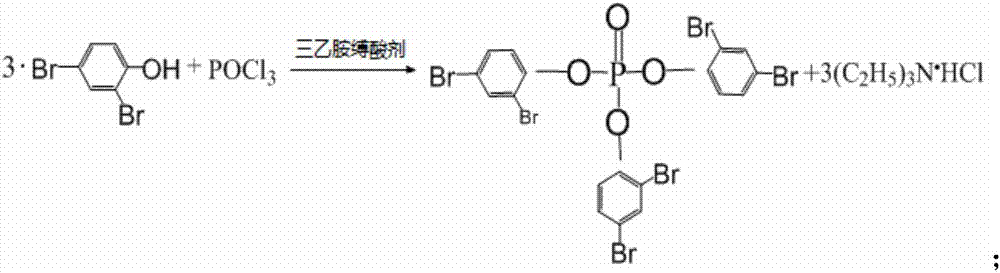
[0050] 2. Synthesis of (2,4-dibromophenyl) phosphate triester
[0051] 1. The above-mentioned product 2,4-dibromophenol chloroform solution, keep the temperature below 25°C, slowly add 60.5g (0.395mol) of...
Embodiment 3
[0061] A method for preparing a plastic flame retardant for flammable hazardous chemicals based on (2,4-dibromophenyl) phosphate triester, comprising the following process steps:
[0062] 1. Synthesis of 2,4-dibromophenol
[0063] 1. In a 2000ml four-necked bottle, add 112.8g (1.2 moles) of industrial phenol, and dissolve it with 1200ml carbon tetrachloride under stirring;
[0064] 2. Control the reaction temperature below 30°C, slowly add 384g (2.4 moles) of industrial bromine dropwise under stirring, and finish the addition in about 2 hours. After the addition, keep the temperature below 30°C and continue the reflux reaction for 1 hour to obtain 2,4- Dibromophenol solution;
[0065] 3. The reaction tail gas is made into hydrobromic acid as a by-product through the water absorption device.
[0066] 2. Synthesis of (2,4-dibromophenyl) phosphate triester
[0067] 1. The above product 2,4-dibromophenol carbon tetrachloride solution, keep the temperature below 25°C, slowly add...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| breaking strength | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com