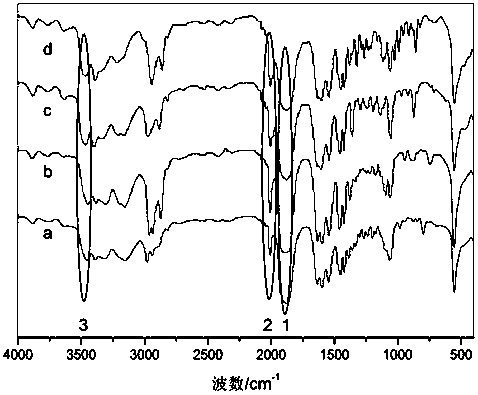
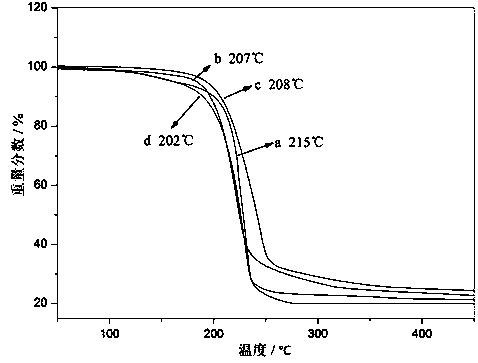
1,1,3,3-tetraalkylguanidine carbonyl cobalt metal organic ionic liquid and its preparation method and application
A technology of tetraalkylguanidine and ionic liquid, which is applied to 1,1,3,3-tetraalkylguanidine carbonyl cobalt metal organic ionic liquid and its preparation and application fields, so as to improve the utilization rate, reduce the production cost and the technological process. easy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Preparation of 1,1,-Dimethyl-3,3-diethylguanidinecarbonyl cobalt metal organic ionic liquid (catalyst a)
[0045] Under N2 protection, KOH (6 mmol) and Co 2 (CO) 8 (1 mmol), then added 6 mL of THF treated with anhydrous and anaerobic treatment, and stirred thoroughly for 2 h. After the reaction was completed, the supernatant was transferred to another Schlenk reaction tube, and then 3 mmol of 1 was added to the Schlenk reaction tube. ,1,-Dimethyl-3,3-diethylguanidine hydrochloride was fully stirred and reacted for 5h. After the reaction was completed, the THF solvent was vacuumed off, and then 6mL of anhydrous and anaerobic treated CH 2 Cl 2 Solution, dissolve and filter to remove precipitate, vacuum remove CH 2 Cl 2 Solvent to obtain the corresponding 1,1,-dimethyl-3,3-diethylguanidine cobalt carbonyl metal organic ionic liquid catalyst a.
[0046] Replace the raw material 1,1,3,3-tetraalkylguanidine hydrochloride with 1,1,-dimethyl-3,3-di-n-butylguanidine di-n-bu...
Embodiment 2
[0049] In an autoclave with a volume of 50 mL, add 3 mL of absolute ethanol, 5 mmol of propylene oxide (Propylene oxide), 2 mol% of 1,1,3,3-tetraalkylguanidine carbonyl cobalt metal organic Ionic Liquid Catalysts a. Seal the reactor, replace the reactor with carbon monoxide 3 times, and seal the reactor. In the Schlenk vacuum line, the reaction system was replaced three times with carbon monoxide gas at room temperature, the pressure of CO gas was filled at 7.0 MPa, and the temperature was slowly raised to 60 by the temperature controller. o C, reacted for 24 hours, cooled to room temperature, unloaded, the liquid obtained by the reaction was carried out qualitative analysis with Agilent 6890 / 5973 gas chromatography spectrometer, and Agilent 7890 gas chromatography was used for quantitative analysis, the conversion rate of propylene oxide was 77%, 3- Ethyl hydroxybutyrate selectivity 90%.
Embodiment 3
[0051] In an autoclave with a volume of 50 mL, add 3 mL of absolute ethanol, 5 mmol of propylene oxide, and 2 mol% of 1,1,3,3-tetraalkylguanidine carbonyl cobalt metal organic ionic liquid catalyst b. Seal the reactor, replace the reactor with carbon monoxide 3 times, and seal the reactor. In the Schlenk vacuum line, the reaction system was replaced three times with carbon monoxide gas at room temperature, the pressure of CO gas was filled at 7.0 MPa, and the temperature was slowly raised to 60 by the temperature controller. o C, reacted for 24 hours, cooled to room temperature, unloaded the kettle, carried out qualitative analysis with Agilent 6890 / 5973 GC-MS, and quantitative analysis with Agilent 7890 gas chromatography, the conversion rate of propylene oxide was 50%, and the 3-hydroxyl Ethyl butyrate selectivity 91%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com