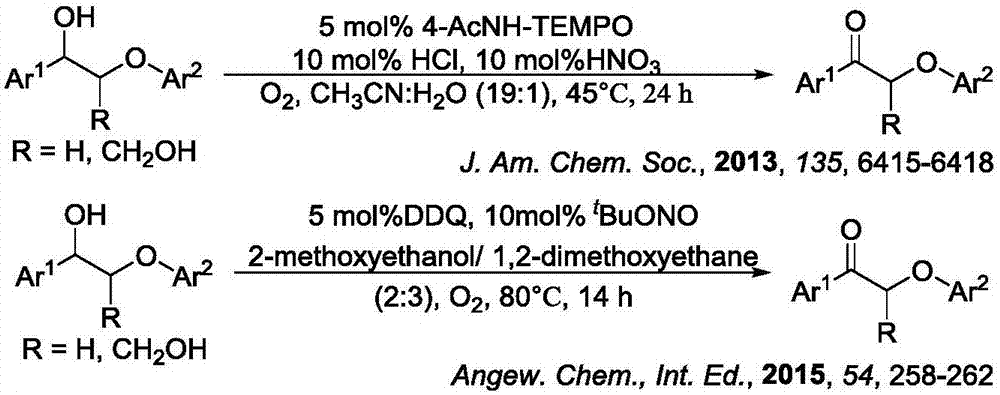
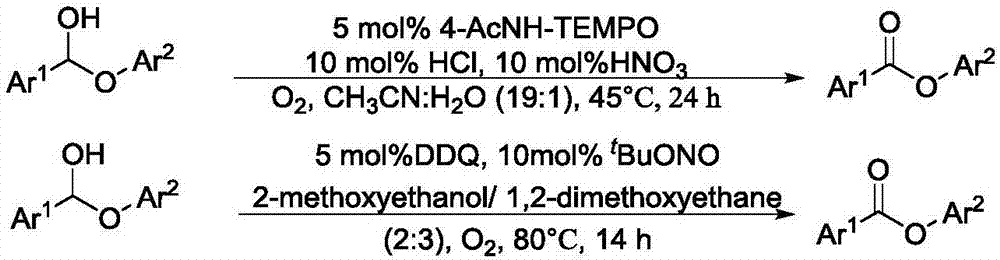
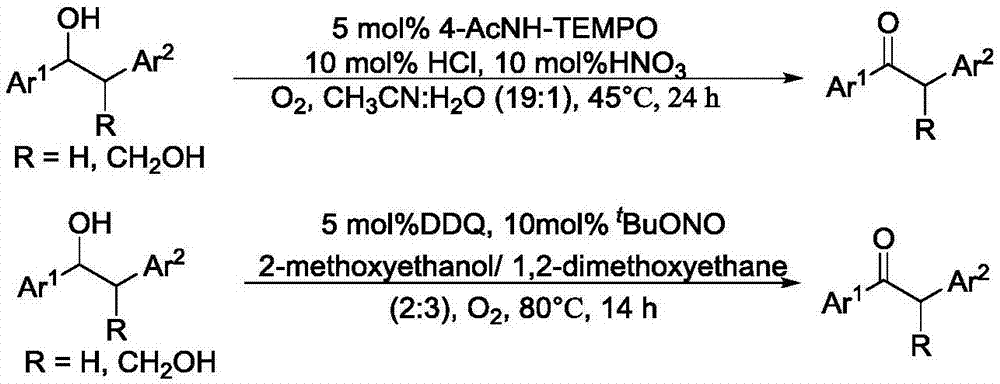
Method for degrading oxidation lignin into small molecular aromatic compound
A technology of aromatic compounds and lignin, which is applied in the field of small molecule aromatic compounds, can solve the problems of harsh catalytic process conditions, metal residues, and unsatisfactory effects, and achieve the effects of convenient operation, high conversion rate, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026]
[0027] (1) Add substrate oxidized lignin dimer 1 (1.211g, 1eq.), oxidant m-chloroperoxybenzoic acid (m-CPBA) (1.73g, 2eq.), and 150mL of methylene chloride to a 250mL round bottom flask. It was dissolved and stirred at room temperature for 12 hours. Column chromatography (petroleum ether) gave acetal 1a (1.12 g, 98% yield) as a colorless oil. NMR data:
[0028] 1 H NMR (500MHz, Chloroform-d) δ8.07 (dd, J = 8.3, 1.3 Hz, 2H), 7.58 (t, J = 7.3 Hz, 1H), 7.44 (t, J = 8.0 Hz, 2H), 7.35 –7.31(m,2H),7.12(d,J=8.5Hz,2H),7.07(t,J=7.3Hz,1H),6.03(s,2H); 13 C NMR(126MHz, CDCl 3 )δ165.6, 157.1, 133.6, 130.0, 129.8, 129.5, 128.6, 122.9, 116.3, 86.4.
[0029] (2) Add step (1) Oxidation product-acetal 1a (0.57g, 2.5mmol) in a 250mL round bottom flask and dissolve it in tetrahydrofuran solvent, add 1mol / L hydrochloric acid solution (0.5mL) as a catalyst, and stir at room temperature for 6 hour. Column chromatography (ethyl acetate: petroleum ether = 1:10, volume ratio) gave the product w...
Embodiment 2
[0033]
[0034] (1) Change the oxidant to hydrogen peroxide (H 2 O 2 ) (2eq.), and other conditions were the same as the step (1) of Example 1, to obtain a colorless oil 1a (1.10g, yield 96%).
[0035] NMR data: as in Example 1.
[0036] (2) Change the solvent to methanol, and other conditions are as in step (2) of Example 1, to obtain colorless liquid methyl benzoate (yield 98%) and colorless liquid phenol (yield 90%).
[0037] NMR data:
[0038] Methyl benzoate 1 H NMR(CDCl 3 ,400MHz): δ8.04(d,J=7.6Hz,2H),7.55(t,J=7.4Hz,1H),7.43(t,J=7.8Hz,2H),3.92(s,3H); 13 C NMR(CDCl 3 ,100MHz): δ167.1, 132.9, 130.1, 129.5, 128.3, 52.0.
[0039] Phenol is as in Example 1.
Embodiment 3
[0041]
[0042] (1) Change the substrate to 2 in the above formula, and other conditions are the same as step (1) of Example 1, to obtain a red oil 2a (yield 30%) and a white solid 2b (yield 65%).
[0043] NMR data:
[0044] 2a 1 H NMR(500MHz, CDCl 3 )δ7.70(dd,J=8.5,2.1Hz,1H),7.51(d,J=2.1Hz,1H),7.13-7.07(m,1H),7.03(td,J=7.8,1.6Hz,1H ), 6.88 (dd, J = 8.2, 1.5 Hz, 1H), 6.83 (dt, J = 9.2, 3.4 Hz, 2H), 6.59 (dd, J = 5.9, 4.1 Hz, 1H), 4.06 (dd, J = 11.9,5.9Hz,1H), 3.98(dd,J=11.9,4.2Hz,1H), 3.88(d,J=6.3Hz,6H), 3.80(s,3H), 3.48(s,1H); 13 C NMR(126MHz, CDCl 3 )δ165.07,153.43,150.77,148.62,145.23,124.72,124.13,121.61,121.04,120.08,112.28,112.11,110.28,97.32,63.17,55.96,55.94,55.77.
[0045] 2b 1 H NMR(500MHz, CDCl 3 )δ7.15–7.02(m,2H), 6.97–6.87(m,2H), 6.81(d,J=8.4Hz,1H), 6.67–6.60(m,2H), 4.90(t,J=4.3Hz ,1H), 4.20(d,J=4.4Hz,2H), 3.89–3.79(m,27H),3.61(s,1H); 13 C NMR(126MHz, CDCl 3 )δ168.64,150.60,149.40,147.08,146.78,143.92,124.04,121.16,118.82,112.66,112.46,111.17,105.53,80.71,63.26,56.17,5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com