A functionalized temperature-sensitive polymer, a preparing method thereof and applications of the polymer
A temperature-sensitive polymer technology, applied in the field of analytical chemistry, can solve the problems of reducing the enrichment reaction rate and efficiency, and achieve the reduction of solid-liquid interface mass transfer resistance and steric hindrance, high enrichment efficiency and selectivity, Reactive Bioorthogonal Effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Embodiment 1, preparation and performance test of functionalized temperature-sensitive polymer
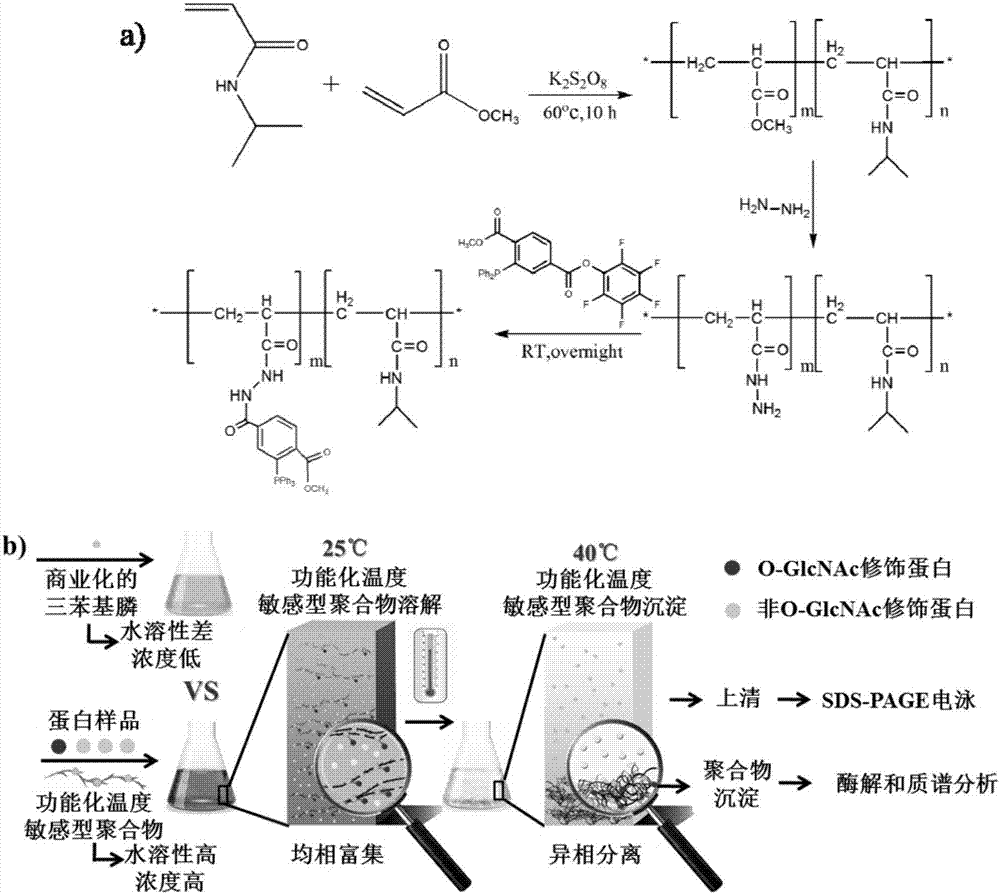
[0062] Synthetic routes for functionalized temperature-sensitive polymers such as figure 1 a) as shown.
[0063] 1) Synthesis of temperature-sensitive polymers: free-radical copolymerization of temperature-sensitive monomers and functional monomers under initiator conditions.
[0064] The details are as follows: add 50mL of 50% methanol to a 100mL round-bottomed flask, stir magnetically and pass N 2 to remove O from the round bottom flask 2 After 1h, weigh 800mg of N-isopropylacrylhydrazide and quickly add it to the above flask, and continue to pass N 2 30min at N 2 Slowly add 0.15mL methyl acrylate under atmosphere, pass N 2 And after stirring for 30min, slowly drop in 80mg of potassium persulfate which has been dissolved in 200μL of deoxygenated water, pass N 2 30min to ensure that the next free radical polymerization reaction is at N 2 Under the atmosphere, remov...
Embodiment 2
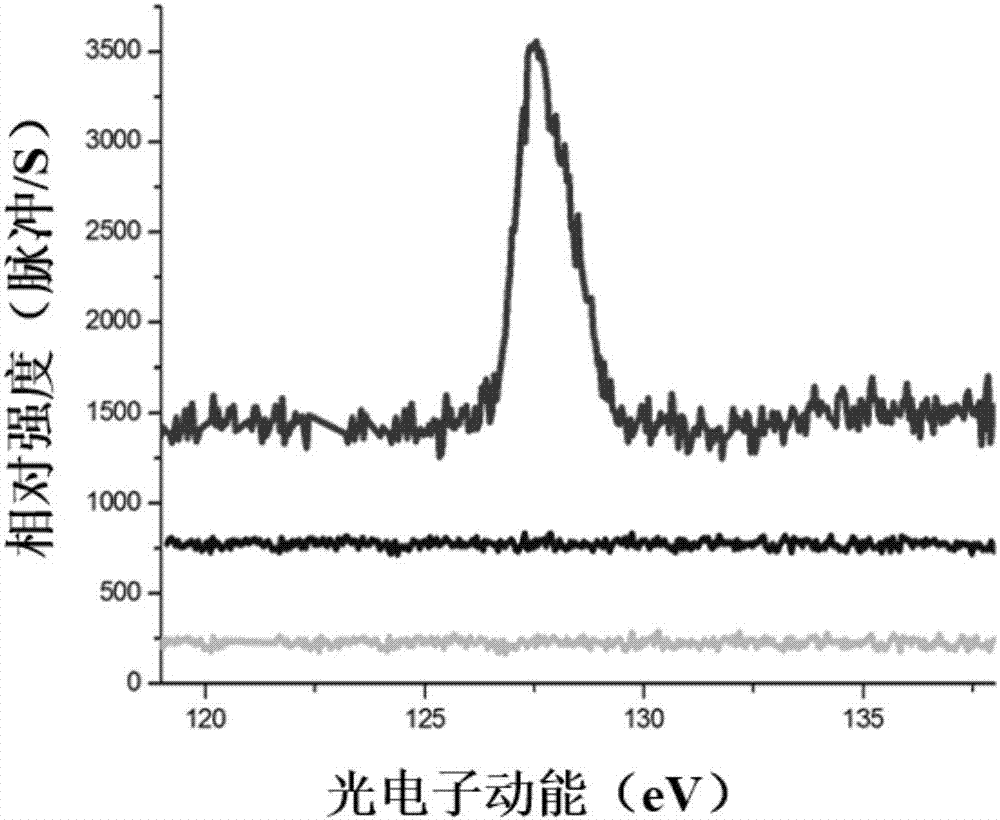
[0089] Example 2. Using the functionalized temperature-sensitive polymer obtained in Example 1 to enrich azide-labeled O-GlcNAc modified proteins
[0090] 1) Weigh an appropriate amount of α-crystallin standard protein and dissolve it in 20mM Hepes buffer solution (pH 7.9) to prepare a concentration of 3.5μgμL -1 standard protein solution. Use the in vitro enzyme catalysis kit to add various reagents according to the instructions to carry out azide labeling on α-crystallin protein overnight at 4°C. After α-crystallin standard protein azido-labeled, methanol, chloroform and deionized water were added in order to precipitate the protein, so as to remove excess UDP-GalNAz, and the excess methanol was blown dry with nitrogen. Finally, the α-crystallin protein was dissolved in 20mM Hepes buffer solution (pH 7.9), and stored at -80°C for later use;
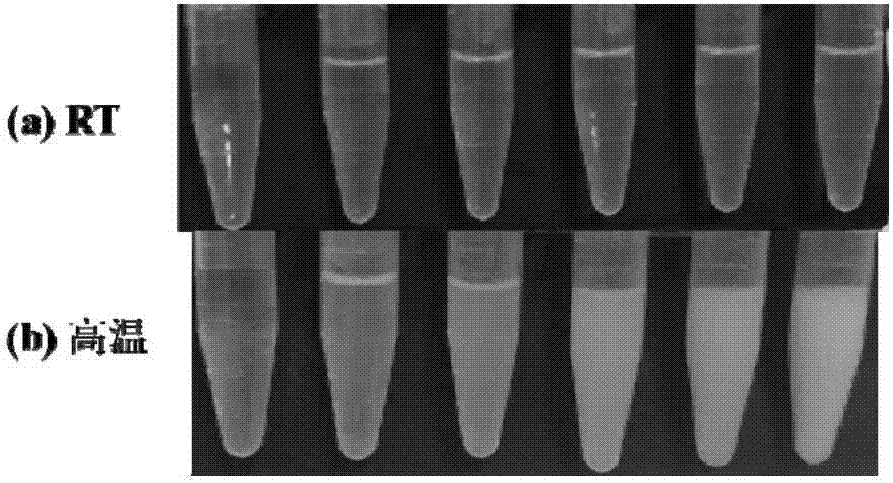
[0091] 2) Weigh 4 parts of functionalized temperature-sensitive polymers, 0.4mg, 0.6mg, 0.8mg and 1.0mg respectively, dissolve in 20...
Embodiment 3
[0094] Example 3. Using the functional temperature-sensitive polymer obtained in Example 1 to enrich O-GlcNAc modified proteins in mixed samples
[0095] 1) Weigh an appropriate amount of α-crystallin standard protein and dissolve it in 20mM Hepes buffer solution (pH 7.9) to prepare a concentration of 3.5μgμL -1 standard protein solution. Use the in vitro enzyme catalysis kit to add various reagents according to the instructions to carry out azide labeling on α-crystallin protein overnight at 4°C. After α-crystallin standard protein azide labeling, add methanol, chloroform, and deionized water in sequence to precipitate the protein, thereby removing excess UDP-GalNAz, and blow dry excess methanol with nitrogen. Finally, the α-crystallin protein was dissolved in 20mM Hepes buffer solution (pH 7.9), and stored at -80°C for later use;
[0096] 2) The mixture of azide-labeled α-crystallin and bovine serum albumin (mass ratio 1:100) was dissolved in 20mM Hepes buffer solution (pH...
PUM
| Property | Measurement | Unit |
|---|---|---|
| transmittivity | aaaaa | aaaaa |
| transmittivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com