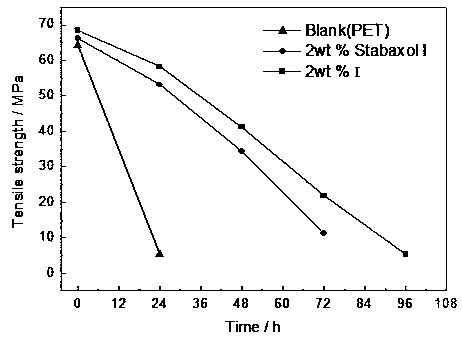
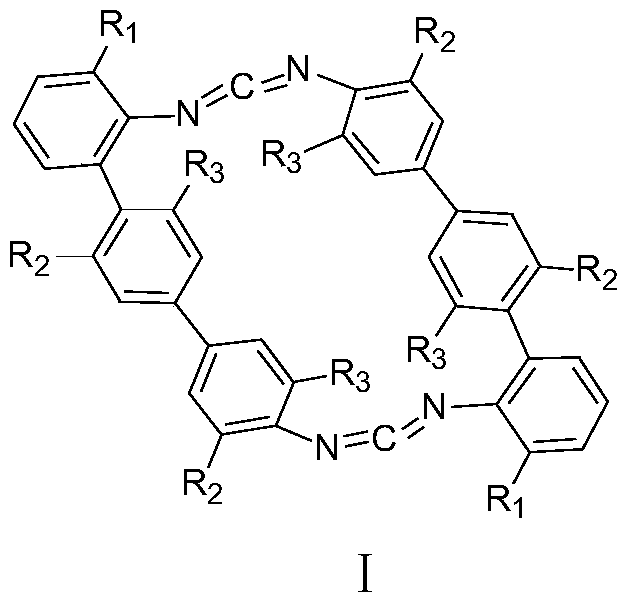
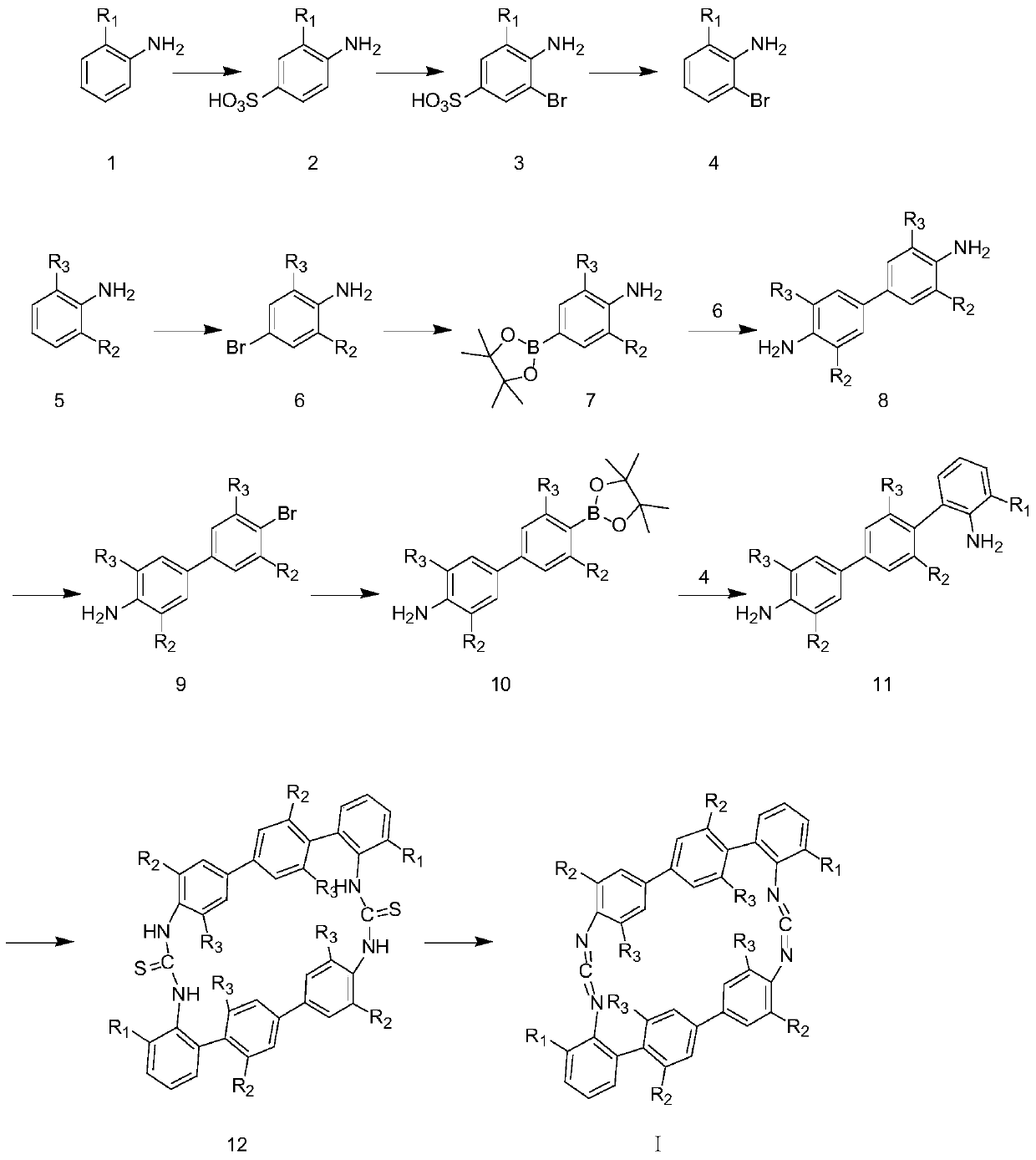
A kind of cyclic biscarbodiimide compound and its preparation method
A biscarbodiimide and compound technology is applied in the field of novel cyclic biscarbodiimide compounds and their preparation, which can solve problems such as deterioration of operating environment, and achieve the effects of easy crystallization and purification, and convenient processing and operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039]1) Add 12mL of concentrated sulfuric acid to 20mL of aqueous solution, heat to 80°C, slowly add 27g of 2-isopropylaniline (1), continue heating and stirring for 15 minutes, then spin evaporate to remove water. Argon atmosphere, heated to 260°C, and continued the reaction for 12h. After cooling down to room temperature, 200mL of 10% aqueous sodium hydroxide solution was added, heated to 90°C, reacted for 30 minutes, cooled and filtered. After the filtrate was adjusted to pH=1 with concentrated sulfuric acid, a large number of solids were precipitated, filtered to obtain 40g sulfoisopropylaniline (2); then in 500mL N,N-dimethylformamide solution, the resulting 40g product (2 ) and 7.2mL of liquid bromine (dissolved in 50mL of N,N-dimethylformamide solution) continued to react for 3.5 hours at room temperature to obtain 35g of brown-red solid bromosulfonic acid isopropylaniline (3); then 35g The product (3) and 300mL of 10% hydrochloric acid were added to a 500mL single-ne...
Embodiment 2
[0046] 1) Add 12mL of concentrated sulfuric acid into 20mL of aqueous solution, heat to 85°C, slowly add 26g of 2-methylaniline (1), continue heating and stirring for 15 minutes, and spin off the water. Argon atmosphere, heated to 250°C, continued the reaction for 13h. After cooling down to room temperature, 200mL of 10% aqueous sodium hydroxide solution was added, heated to 90°C, reacted for 30 minutes, cooled and filtered. After adjusting the filtrate to pH=1 with concentrated sulfuric acid, a large number of solids were precipitated, filtered to obtain 45g of sulfonic acid methylaniline (2); And 9.0mL liquid bromine (dissolved in 50mL N,N-dimethylformamide solution) continued to react at room temperature for 4 hours to obtain 40g brownish red solid bromosulfonic acid methylaniline (3); then 40g product ( 3) and 350mL of 11% hydrochloric acid were added to a 500mL single-necked bottle, heated to reflux for 11 hours, cooled to room temperature, neutralized with saturated sod...
Embodiment 3
[0052] 1) Add 12mL of concentrated sulfuric acid to 20mL of aqueous solution, heat to 80°C, slowly add 27g of 2-tert-butylaniline (1), continue heating and stirring for 15 minutes, then spin evaporate to remove water. Argon atmosphere, heated to 270°C, and continued to react for 12h. After cooling down to room temperature, 150 mL of 10% aqueous sodium hydroxide solution was added, heated to 90°C, reacted for 30 minutes, cooled, and filtered. After the filtrate was adjusted to pH=1 with concentrated sulfuric acid, a large number of solids were precipitated, filtered to obtain 54g sulfonic acid tert-butylaniline (2); then in 500mL N,N-dimethylformamide solution, the resulting 54g product (2 ) and 9.9mL liquid bromine (dissolved in 50mL N,N-dimethylformamide solution) continued to react for 4 hours at room temperature to obtain 52g brownish red solid bromosulfonic acid tert-butylaniline (3); then 52g The product (3) and 300mL of 10% hydrochloric acid were added to a 500mL single...
PUM
| Property | Measurement | Unit |
|---|---|---|
| tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com