Copper powder and electrically conductive paste, electrically conductive coating, electrically conductive sheet, and antistatic coating using same
A technology of conductivity and copper powder, which is applied in the direction of conductive coatings, conductive materials, conductive materials dispersed in non-conductive inorganic materials, etc., can solve the problems of unsuitable use of metal fillers, increased viscosity of pastes, and inability to disperse uniformly. Achieve excellent uniform dispersion, suppress viscosity increase, and high electrical conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0118] In an electrolytic cell with a capacity of 100L, a titanium electrode plate with an electrode area of 200 mm × 200 mm is used as a cathode, and a copper electrode plate with an electrode area of 200 mm × 200 mm is used as an anode. Enter the electrolyte, connect it with direct current, and make the copper powder precipitate on the cathode plate.
[0119] At this time, as the electrolytic solution, an electrolytic solution having a composition having a copper ion concentration of 10 g / L and a sulfuric acid concentration of 100 g / L was used. In addition, polyethylene glycol (PEG) with a molecular weight of 400 (manufactured by Wako Pure Chemical Industries, Ltd.) was added as an additive to the electrolytic solution so that its concentration in the electrolytic solution became 500 mg / L, and further, hydrochloric acid was added A solution (manufactured by Wako Pure Chemical Industries, Ltd.) was prepared so that the concentration of chloride ions (chloride ions) was 50...
Embodiment 2
[0125] In the electrolytic solution, PEG with a molecular weight of 400 was added as an additive so that the concentration became 1000 mg / L, and furthermore, a hydrochloric acid solution was added so that the concentration of chloride ions became 50 mg / L. 1 The same conditions cause copper powder to precipitate on the cathode plate.
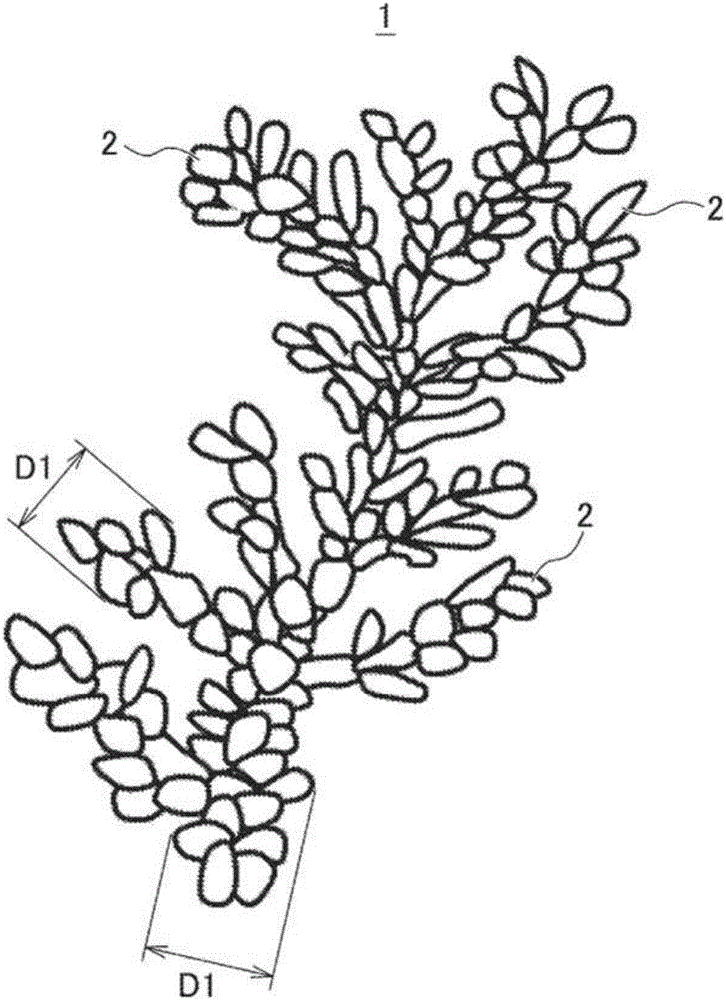
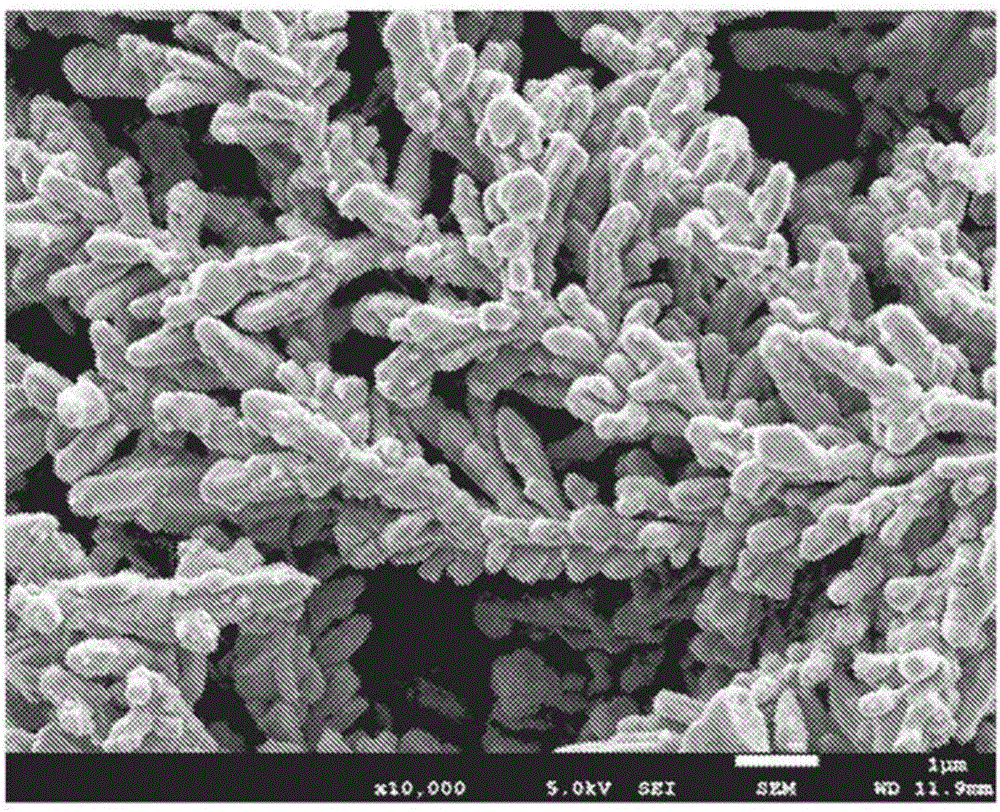
[0126] The shape of the obtained electrolytic copper powder was observed by the method based on the scanning electron microscope (SEM). As a result, the deposited copper powder formed a dendritic shape in which elliptical copper particles were aggregated. The elliptical copper particles are elliptical copper particles having a diameter of 0.2 μm to 0.5 μm and an average value of 0.32 μm, and a length of 0.5 μm to 2.0 μm and an average value of 1.4 μm. In addition, the crystallite diameter of the elliptical copper particles is
[0127] In addition, the average particle diameter of the dendritic copper powder formed by aggregating the elliptical...
Embodiment 3
[0129] so that the current density of the cathode becomes 10A / dm 2 In addition, the same conditions as in Example 1 were used to deposit copper powder on the cathode plate.
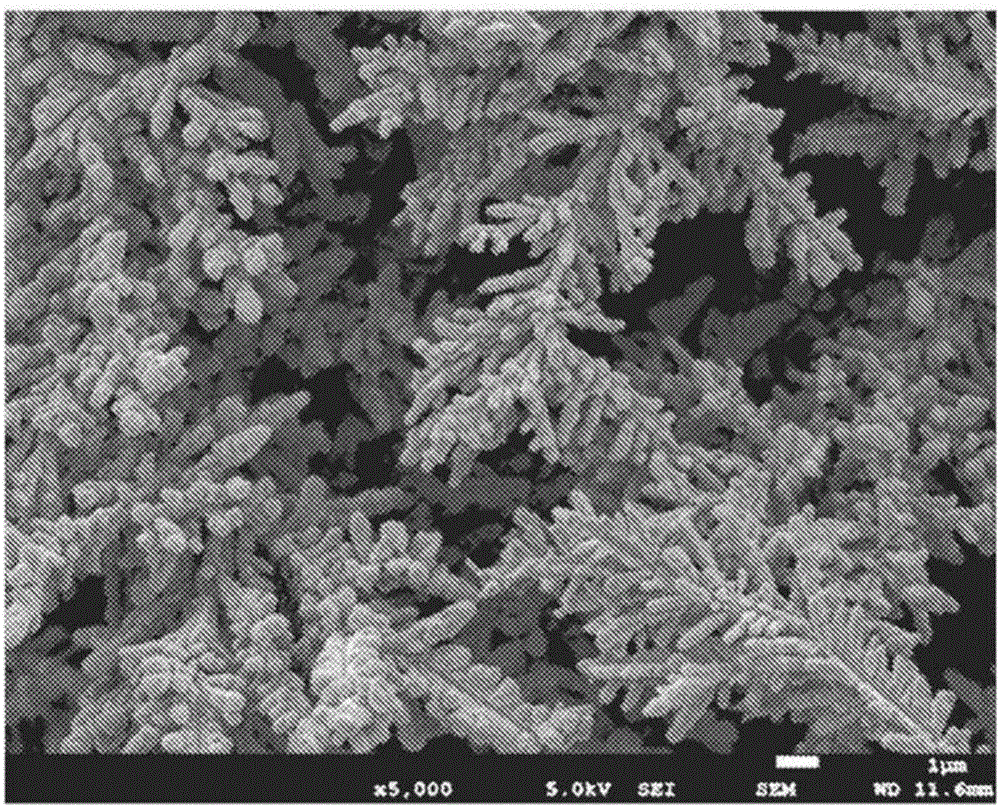
[0130] Observation of the shape of the obtained electrolytic copper powder by the method based on the above-mentioned scanning electron microscope (SEM) revealed that the precipitated copper powder had a dendritic shape in which elliptical copper particles were aggregated. The copper particles are elliptical copper particles having a diameter of 0.2 μm to 0.5 μm and an average value of 0.48 μm, and a length of 0.5 μm to 2.0 μm and an average value of 1.8 μm. In addition, the crystallite diameter of the elliptical copper particles is
[0131] In addition, the average particle diameter of the dendritic copper powder formed by aggregating the elliptical copper particles was 18.2 μm. In addition, it was confirmed that the dendritic copper powder having a thickness (diameter) of the dendritic portion of 0....
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com