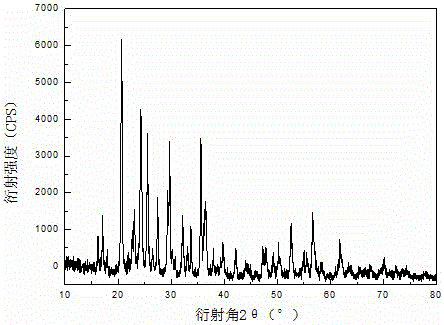
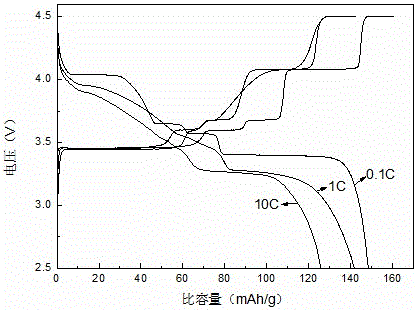
Preparation method of lithium iron phosphate-lithium vanadium phosphate flaky composite cathode material
A composite positive electrode material, lithium iron phosphate technology, applied in the direction of chemical instruments and methods, phosphorus compounds, battery electrodes, etc., can solve the problems of reduced electron mobility, poor low temperature performance and rate performance, low electronic conductivity, etc., to achieve excellent Electronic conductivity, ionic conductivity, and the effect of improving rate performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Weigh 9.36g (0.08mol) of ammonium metavanadate, 3.19g (0.02mol) of ferric oxide, 18.40g (0.16mol) of ammonium dihydrogen phosphate, 5.91g (0.08mol) of lithium carbonate, and 7.20g (0.08mol) of oxalic acid mol), according to the molar ratio of vanadium atom, iron atom, phosphorus atom, lithium atom, carbon atom is 2:1:4:4:4 dissolved in 1L deionized water, and then according to the theoretical obtained lithium iron phosphate-lithium vanadium phosphate mass Add 0.678g of surfactant cetyltrimethylammonium bromide at 3%, control the concentration of vanadium ions in the solution to 0.08mol / L, adjust the pH value to 6 with ammonia water, stir with a magnetic stirrer for 11h, and obtain a uniform transparent solution ;Transfer the obtained homogeneous transparent solution to a high-pressure reactor, feed argon, react at a speed of 900rpm and a temperature of 250°C for 20h, wash, filter, dry, and grind to obtain lithium iron phosphate-vanadium phosphate Lithium composite mater...
Embodiment 2
[0028] Take 5.46g (0.03mol) of vanadium pentoxide, 4.32g (0.03mol) of ferrous oxalate, 12.47g (0.12mol) of lithium dihydrogen phosphate, and 3.36g (0.01075mol) of citric acid. Atoms, lithium atoms, and carbon atoms in a molar ratio of 2:1:4:4:3.5 were dissolved in 1L of deionized water, and then the surfactant polydioctyl succinate was added according to 1% of the theoretically obtained lithium iron phosphate-lithium vanadium phosphate mass. Sodium ester sulfonate 0.170g, control the vanadium ion concentration in the solution to be 0.06mol / L, adjust the pH value to 5 with ammonia water, stir with a magnetic stirrer for 10h, and obtain a homogeneous solution transparent solution; the obtained homogeneous transparent solution is transferred to a high pressure In the reaction kettle, a protective gas was introduced to react at a speed of 800 rpm and a temperature of 240°C for 25 hours. After washing, filtering, drying, and grinding, the precursor powder of lithium iron phosphate-l...
Embodiment 3
[0031] Weigh 16.55g (0.09mol) of sodium orthovanadate, 18.18g (0.045mol) of iron nitrate, 18.71g (0.18mol) of lithium dihydrogen phosphate, 5.78g (0.017mol) of sucrose , lithium atom, carbon atom molar ratio is 2:1:4:4:4.5 dissolved in deionized water, and then add surfactant octylphenyl polyvinyl ether according to 5% of the mass of lithium iron phosphate-lithium vanadium phosphate obtained theoretically 1.27g, control the concentration of vanadium ions in the solution to be 0.09mol / L, adjust the pH value to 7 with ammonia, stir with a magnetic stirrer for 12h, and obtain a homogeneous solution transparent solution; the obtained homogeneous transparent solution is transferred to an autoclave, Introduce protective gas, react at a speed of 1000rpm and a temperature of 260°C for 15 hours, wash, filter, dry, and grind to obtain a precursor powder of lithium iron phosphate-lithium vanadium phosphate composite material; Calcined at 780° C. for 15 hours in a non-oxidizing atmosphere...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com