Door control type medicine composition integrating cancer imaging and phototherapy and preparation method
A drug compound and imaging technology, which is applied in the field of biomedicine and achieves the effects of simple preparation process, low price and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
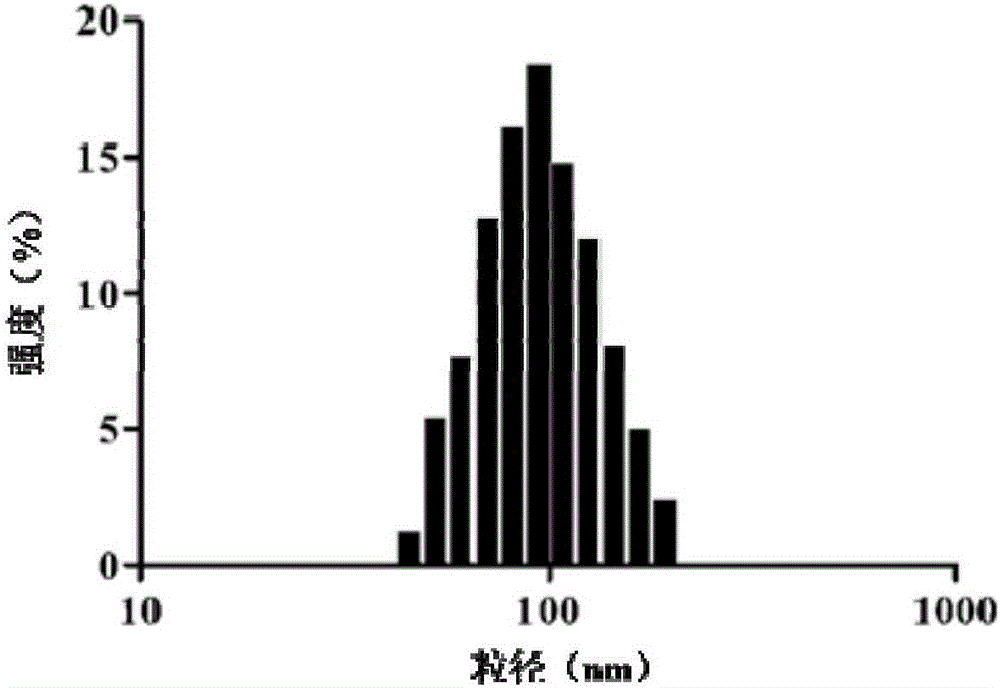
Image
Examples
Embodiment 1
[0041] A method for preparing a gated drug complex integrating cancer imaging and phototherapy, comprising the following steps:
[0042] (1) Core-shell structure CuS@mSiO 2 Preparation of nanoparticles:
[0043] A. The proportion, the 13mgCuCl 2 and 24 mg Na 2 S·9H 2 O was added to 100 mL of 0.13 mg / mL cetyltrimethylammonium bromide (CTAB) aqueous solution to obtain a brown solution, stirred at room temperature for 18 minutes, and heated at 90°C under nitrogen protection to make the color change. Turn into dark green, continue to react for 60 minutes, cool to 70°C, then add cetyltrimethylammonium bromide to make the total concentration of cetyltrimethylammonium bromide 2mg / mL, stir for 16 minutes, Adjust the pH to 10, add 400 μL tetraethyl orthosilicate (TEOS) and 2 mL ethyl acetate, react for 28 minutes, add 130 μL silane-modified PEG with a weight average molecular weight of 700-3000, react for 130 minutes, centrifuge, and precipitate with absolute ethanol wash 3 times; ...
Embodiment 2
[0053] Temperature-mediated ICG release experiments:
[0054] In this experiment, an external water bath was used to adjust the required temperature. First, 5 mg of CuS@mSiO prepared in Example 1 2 -TD / ICG was dispersed into 2 mL of ultrapure water, and the dispersion was placed in a dialysis bag, and the whole was placed in a phosphate buffered saline release medium with a pH of 7.4. We choose two temperature modes: constant temperature mode and beating variable temperature mode. In the constant temperature mode, we compared CuS@mSiO at room temperature (25°C), 37°C and 45°C 2 - Release amount of ICG in TD / ICG; In the variable temperature mode, choose 37°C and 45°C alternately as the heat source to observe the drug release behavior.
[0055] Such as image 3 As shown in a, at room temperature and 37 °C, the release amount of ICG is very small, because the temperature at this time is lower than the phase transition temperature of TD, it is in a solid state, and ICG cannot ...
Embodiment 3
[0057] Test CuS@mSiO 2 -TD / ICG photothermal heating curve:
[0058] Take 1ml of CuS@mSiO 2 -TD / ICG aqueous solution (CuS@mSiO 2 The concentration is 200μg / mL, while the ICG concentration is 10μg / mL) into a 1.5mL EP tube, use 808nm, 1.5W / cm 2 The power laser was irradiated for 5 minutes, and the temperature change within 0-5 minutes was recorded using a temperature monitor equipped with a thermocouple microprobe (φ=0.5mm). Such as Figure 4 Shown is CuS@mSiO 2 -The photothermal heating curve of TD / ICG, it can be seen that the prescription can rapidly raise the temperature under light to achieve the purpose of photothermal therapy.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com