A peptide-based osteoblast targeting carrier and its preparation and application
An osteoblast-targeted technology, applied in medical preparations with non-active ingredients, medical preparations containing active ingredients, bone diseases, etc., can solve potential toxicity and carcinogenicity, can not eliminate biological effects, very Difficult to eliminate and other problems, to achieve the effects of clear biomineralization ability, low production cost, strong plasticity and low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
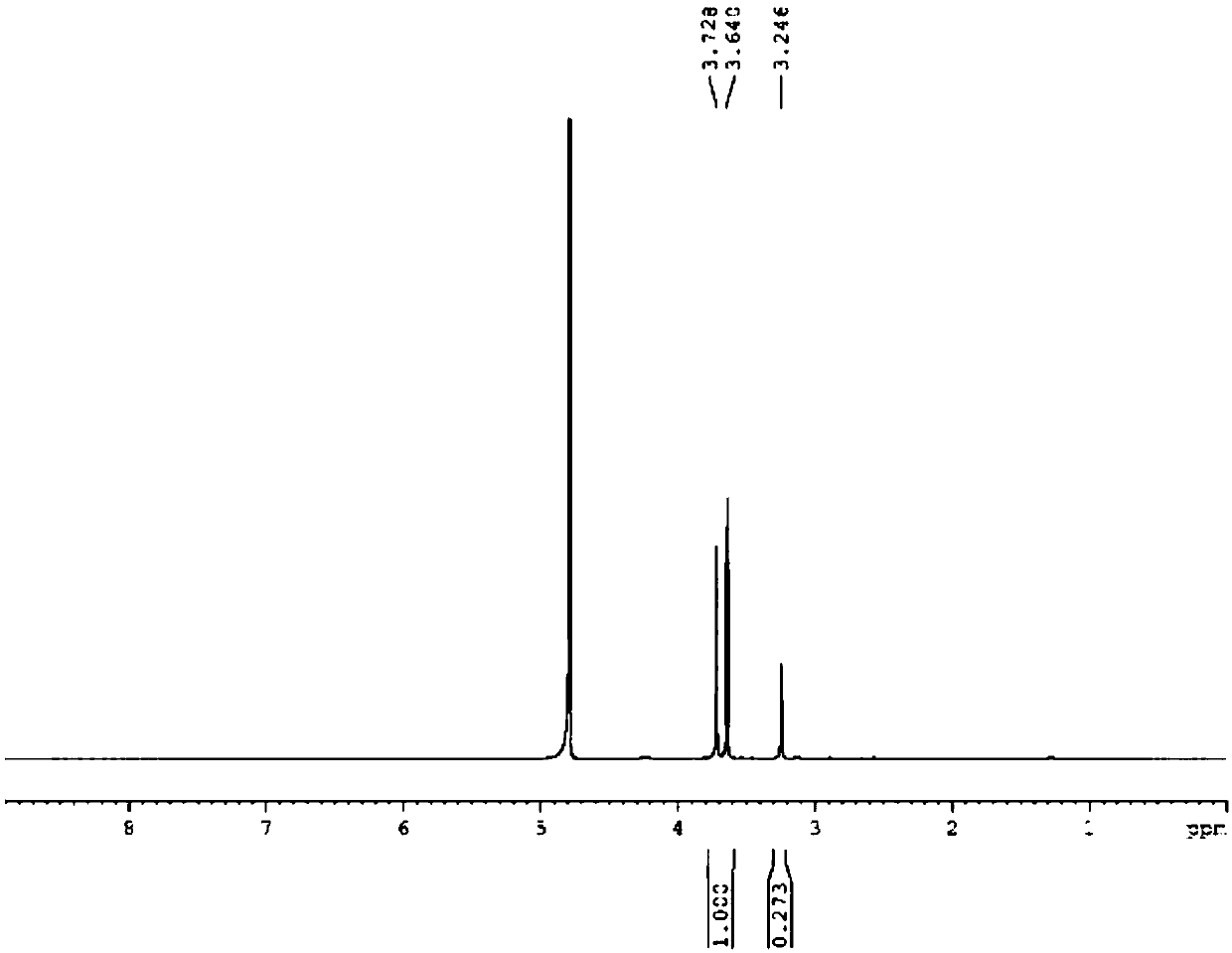
[0046] An osteoblast targeting carrier constructed based on a polypeptide, the targeting carrier includes a target head and a scaffold, and the target head and the scaffold are covalently connected to the target head and the scaffold through polyethylene glycol carboxylic acid (PEG-COOH). The mass coverage rate of the target head is about 6%, and the molar coverage rate of the target head is 3%. The target head is a polypeptide composed of serine and aspartic acid, wherein the sequence of the polypeptide is Ser AspSer Ser Asp, wherein Ser represents serine and Asp represents aspartic acid; the scaffold is a polyurethane scaffold.
[0047] The above-mentioned targeting carrier, its synthesis method is as follows: figure 1 shown, including the following steps:
[0048] (1) Preparation of the target head: using a twelve-channel semi-automatic peptide synthesizer, the target head was synthesized by using the amino acid condensation sequence connection method, and serine (Fmoc-Asp...
Embodiment 2
[0059] An osteoblast targeting carrier constructed on the basis of polypeptides, the targeting carrier includes a target head and a scaffold, and the target head and the scaffold are covalently connected through polyethylene glycol carboxylic acid (PEG-COOH). The mass coverage rate of the target head is about 5%. The target head is a polypeptide composed of serine and aspartic acid, wherein the sequence of the polypeptide is Ser Asp, wherein Ser represents serine, and Asp represents aspartic acid; the scaffold is a polyurethane scaffold.
[0060] The synthesis method of the above-mentioned targeting carrier, for example, comprises the following steps:
[0061] (1) Preparation of the target head: using a twelve-channel semi-automatic peptide synthesizer, the target head was synthesized by using the amino acid condensation sequence connection method, and serine (Fmoc-Asp(otBu)-OH) and aspartic acid (Fmoc-Ser(Tbu) )-OH) is connected in the order of Ser-Asp to obtain the target h...
Embodiment 3
[0067] An osteoblast targeting carrier constructed based on a polypeptide, the targeting carrier includes a target head and a scaffold, and the target head and the scaffold are covalently connected through polyethylene glycol carboxylic acid (PEG-COOH). The grafting rate of the target head mass is about 6%. The target head is a polypeptide composed of serine and aspartic acid, with a length of 20 amino acids, wherein the sequence of the polypeptide is Ser Asp Asp Asp AspSer Asp Asp Asp Asp Ser Asp Asp Asp Asp Ser Asp Asp Asp Asp(Ser:Asp= 1:4), wherein, Ser represents serine, Asp represents aspartic acid; the scaffold is a polyurethane scaffold.
[0068] The synthesis method of the above-mentioned targeting carrier, for example, comprises the following steps:
[0069](1) Preparation of the target head: using a twelve-channel semi-automatic peptide synthesizer, the target head was synthesized by using the amino acid condensation sequence connection method, and serine (Fmoc-Asp(...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| degree of grafting | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com