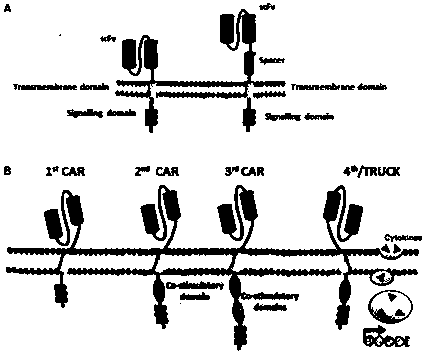
A replication-deficient recombinant lentiviral car-t transgene vector targeting CD123 and its construction method and application
A technology of recombinant lentivirus and transgenic vector, applied in the field of medical biology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
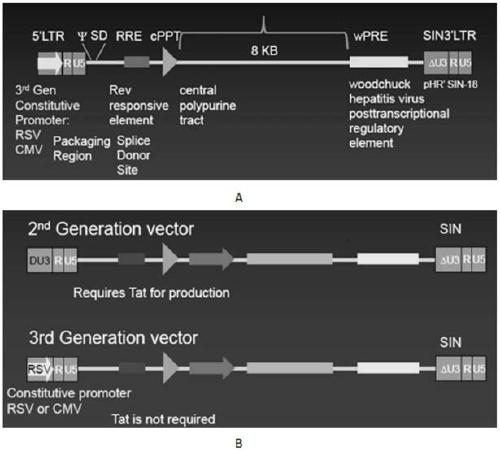
[0067] Example 1 Construction of recombinant lentiviral vector
[0068] 1. Materials
[0069] 1. The lentiviral backbone plasmid pLenti-3G basic, the lentiviral packaging plasmids pPac-GP, pPac-R and the membrane protein plasmid pEnv-G, HEK293T / 17 cells, and homologous recombination enzyme were supplied by Shiao (Shanghai) Biomedical Technology Co., Ltd. supply;
[0070] 2. Primers: According to the primer design principles, design the primers required to amplify the DNA fragments and target sites. The primers are synthesized by Shanghai Biological Company, specifically:
[0071] EF1α-F: 5'-ATTCAAAATTTTATCGATGCTCCGGTGCCCGTCAGT-3' (SEQ ID NO.26)
[0072] EF1α-R: 5'-TCACGACACCTGAAATGGAAGA-3' (SEQ ID NO.27)
[0073] CD8leader-F: 5'-GGTGTCGTGAGGATCCGCCACCATGGCCTTACCAGTGACCGC-3' (SEQ ID NO.28)
[0074] CD8leader-R: 5'-GTGTCATCTGGATGTCCGGCCTGGCGGCGTG-3' (SEQ ID NO.29)
[0075] VH-F: 5'-CACGCCGCCAGGCCGGAAGTGAAACTGGTGGAAAGC-3' (SEQ ID NO.30)
[0076] VH-R: 5'-GCTGCTCACGGTCACCA...
Embodiment 2
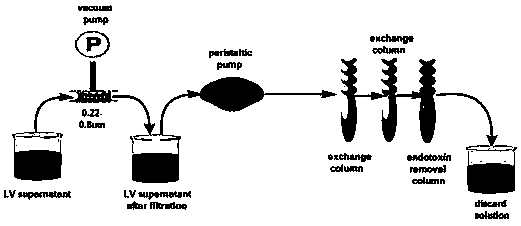
[0158] Example 2 Concentration and detection of recombinant lentiviral vector
[0159] 1. Purification of recombinant lentiviral vector by ultracentrifugation;
[0160] (1) Divide the collected supernatant into 50ml centrifuge tubes, centrifuge at 500g room temperature for 10min, and remove cells and large debris;
[0161] (2) Filter the supernatant with a 0.22 μm-0.8 μm filter;
[0162] (3) Take 6 Hitachi 40PA ultracentrifuge tubes, spray 70% ethanol on the surface to sterilize them, put them on a clean table and irradiate them with ultraviolet light for 30 minutes to sterilize them. It can also be sterilized by high temperature and moist heat;
[0163] (4) Aliquot 32ml of the cell supernatant sample processed in step 2 into a centrifuge tube;
[0164] (5) Cover the metal cover, balance the centrifuge tube together with the metal cover, and adjust with 1XPBS to make the weight deviation within 0.02g;
[0165] (6) Place the balanced centrifuge tubes symmetrically in the ul...
Embodiment 3
[0242] Example 3 Functional detection of recombinant lentiviral vectors lvCAR123-CLA, lvCAR123-CLB, and lvCAR123-OLC.
[0243] 1. Cell-level expression detection of CAR gene:
[0244] (1) After infecting PBMC cells with recombinant lentiviral vectors lvCAR123-CLA, lvCAR123-CLB, and lvCAR123-OLC, collect the cells and detect CAR mRNA transcription levels by RT-PCR to verify the expression of CAR genes. If the CAR mRNA transcription levels increase, then It shows that the transcription level expression of CAR gene is successful;
[0245] (2) After infecting PBMC cells with recombinant lentiviral vectors lvCAR123-CLA, lvCAR123-CLB, and lvCAR123-OLC, collect the cells and detect the expression level of CAR protein by western blot to verify the expression of CAR gene. If the expression level of CAR protein increases, then It shows that the translation level expression of CAR gene is successful;
[0246] (3) Infect the cells with lvCAR123-CLA, lvCAR123-CLB, lvCAR123-OLC and the co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com