Pigeon paramyxovirus 1 strain AF-1 and application thereof
An AF-1, paramyxovirus technology, applied in the direction of viruses, antiviral agents, virus antigen components, etc., can solve the problems of unsatisfactory protection effect and genotype differences, and achieve good safety, good immunogenicity, and multiplication droplets. high degree of effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
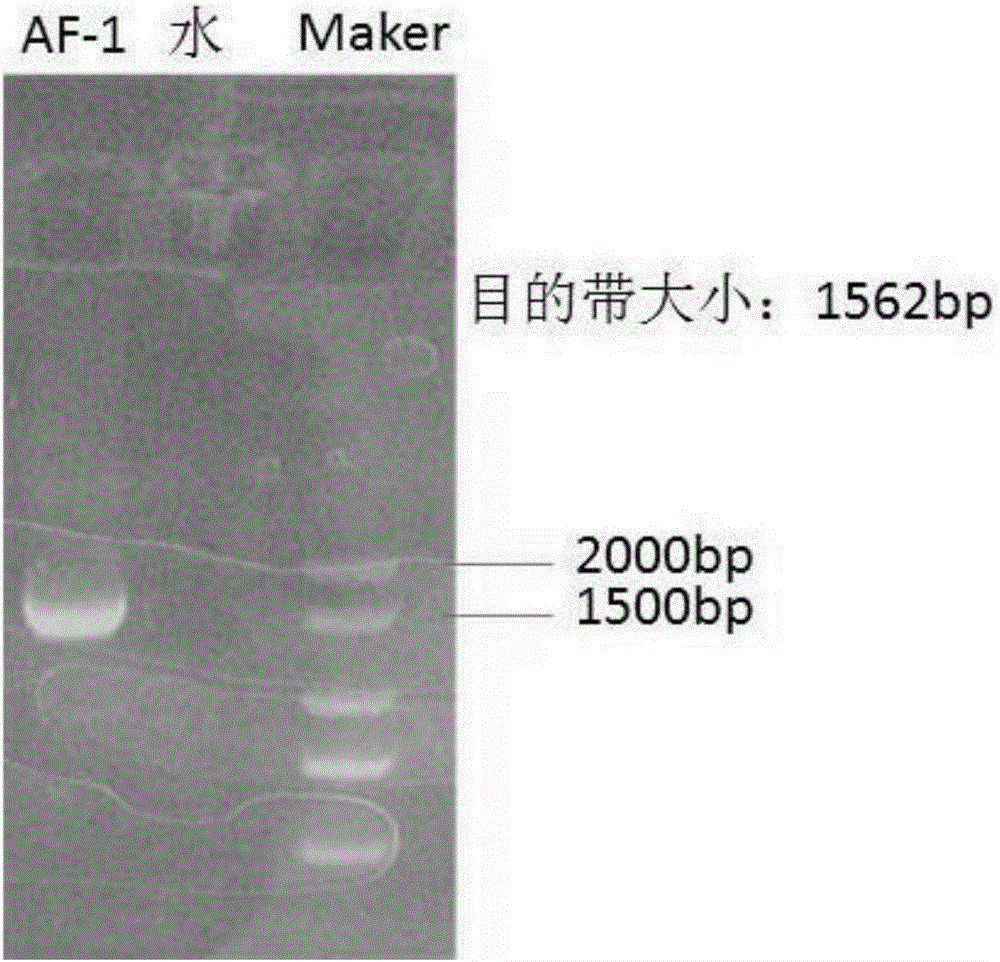
[0022] The isolation and identification of embodiment 1 virus
[0023] The visceral tissues of dead pigeons were collected from a pigeon farm in Anhui, and after RNA extraction and detection by RT-PCR, it was determined that they were pigeon paramyxovirus type 1, and the virus strain was named as pigeon paramyxovirus PPMV-1AF-1 strain. The specific operation steps are as follows:
[0024] 1. Chicken embryo multiplication
[0025] Add double antibody (final concentration: 10000U / ml) to the disease material treatment solution, incubate at 33-35°C for 30 minutes, inoculate 9-11-day-old SPF chicken embryos through the chorioallantoic cavity, and inoculate 4 chicken embryos per disease material 0.2ml per embryo. The inoculated chicken embryos were incubated at 33-35°C for 96 hours, the humidity was maintained at 60-65%, and the eggs were illuminated regularly. Chicken embryos that died within 24 hours were discarded. Take the dead and surviving chicken embryos that have been de...
Embodiment 2
[0045]The preparation of embodiment 2 inactivated vaccine 1
[0046] 1. Determination of virus content
[0047] The virus liquid was diluted serially 10 times with sterilized physiological saline, and 10 -6 、10 -7 、10 -8 Inoculate 10-day-old SPF chicken embryos into the allantoic cavity of 3 dilutions, inoculate 5 eggs for each dilution, 0.1ml / embryo, record the infection status of chicken embryos in 24-120 hours, and calculate EID 50 , the virus content per 0.1ml should be ≥10 7.0 EID 50 . After determination, the EID after harvesting the allantoic fluid was mixed 50 See the table below:
[0048] Table 1 Reed-Muench method to calculate EID 50 result
[0049]
[0050] The calculation method is as follows:
[0051]
[0052] wxya 50 = logarithm of highest dilution of virus above 50% infection + distance ratio x difference between dilutions.
[0053] Distance Scale = 0.78
[0054] wxya 50 =-7+(-1×0.78)=-7.78
[0055] EID 50 =10 -7.78 / 0.1ml
[0056] 2. Vir...
Embodiment 3
[0068] 1. Vaccine safety test
[0069] With 15 paramyxovirus type I antibody-negative pigeons at the age of 21 to 30 days, 10 of them were subcutaneously injected with 1 ml of vaccine in each neck, and the other 5 were used as controls. After 14 days of observation, there should be no local and systemic adverse reactions caused by the injection of the vaccine. The test results are shown in the table:
[0070] Table 3 Safety detection of paramyxovirus type I antibody-negative pigeons in 21-30 days old pigeons inoculated with overdose of inactivated vaccine
[0071] Before vaccination after inoculation immune group 10 / 10 normal 10 / 10 normal control 5 / 5 OK 5 / 5 OK
[0072] 2. Immunity and virus attack protection experiment
[0073] 2.1. Immunity
[0074] With 15 paramyxovirus type I antibody-negative pigeons at the age of 21 to 30 days, 10 pigeons were subcutaneously injected with 200 μl of vaccine in each neck, and the other 5 were used as ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com