Thiophene and pyrrole quinoid compound and preparation method thereof
A technology of thiophene quinone compound, which is applied in the field of preparation of thienopyrrole quinone compound, to achieve narrow band gap, strong light absorption ability, and beneficial to solution processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
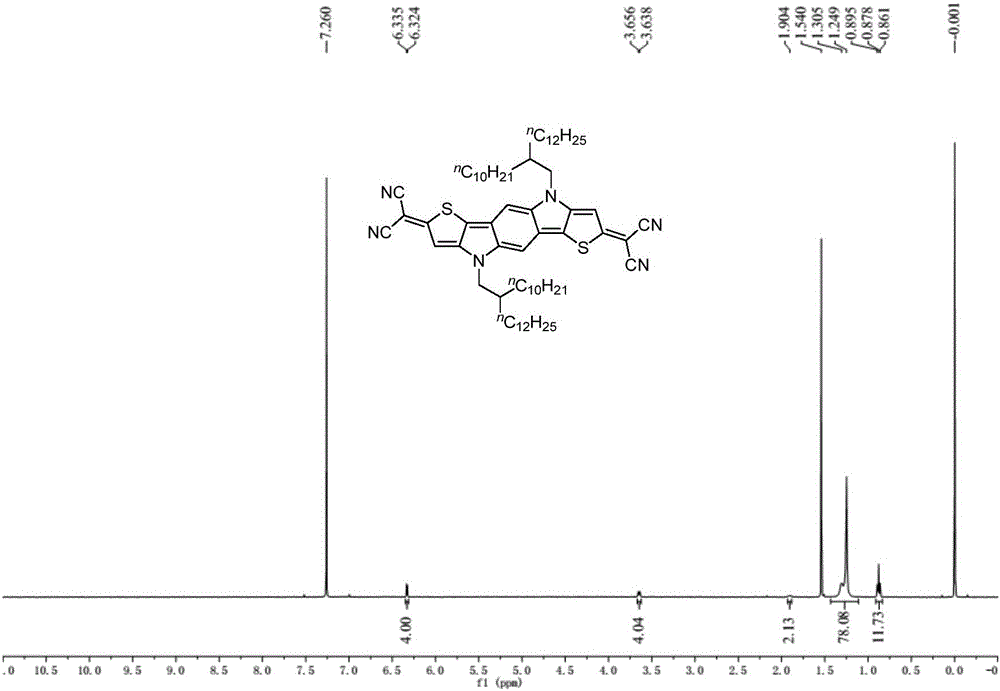
[0053] The preparation of embodiment 1 compound Ia
[0054]
[0055] Under nitrogen protection at -78°C, n-butyl lithium (1.6M in hexane, 188 μL, 0.30 mmol) was slowly added dropwise to a 10 mL three-necked flask containing compound IVa (113.0 mg, 0.12 mmol) and tetrahydrofuran (2 mL), Keep the reaction at -78°C for 30 minutes, add elemental iodine (76.1mg, 0.30mmol), rise to room temperature and react for 2 hours, add saturated sodium thiosulfate solution (5mL) to quench, and extract with ether (30mL×3), The organic phases were combined and washed with saturated brine, dried over anhydrous magnesium sulfate, and the solvent was removed by rotary evaporation to obtain the crude compound IIa, which was directly used in the next reaction.
[0056] At 0°C, under nitrogen protection, malononitrile (39.6 mg, 0.60mmol), after the foam disappeared, it was raised to room temperature and reacted for 30 minutes. The resulting malononitrile anion solution was transferred via cannula...
Embodiment 2
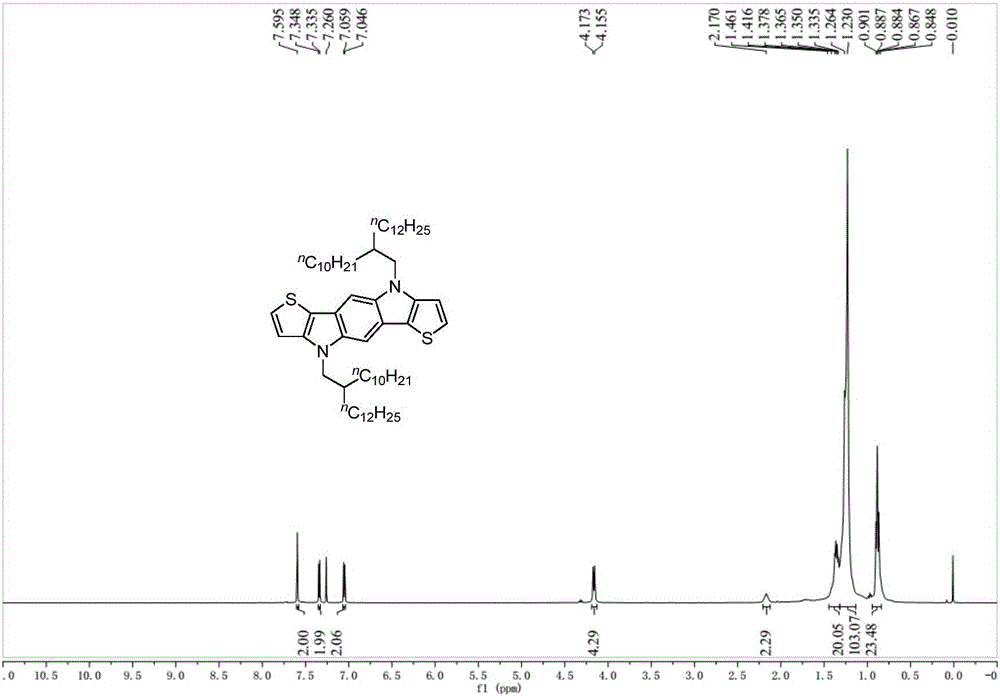
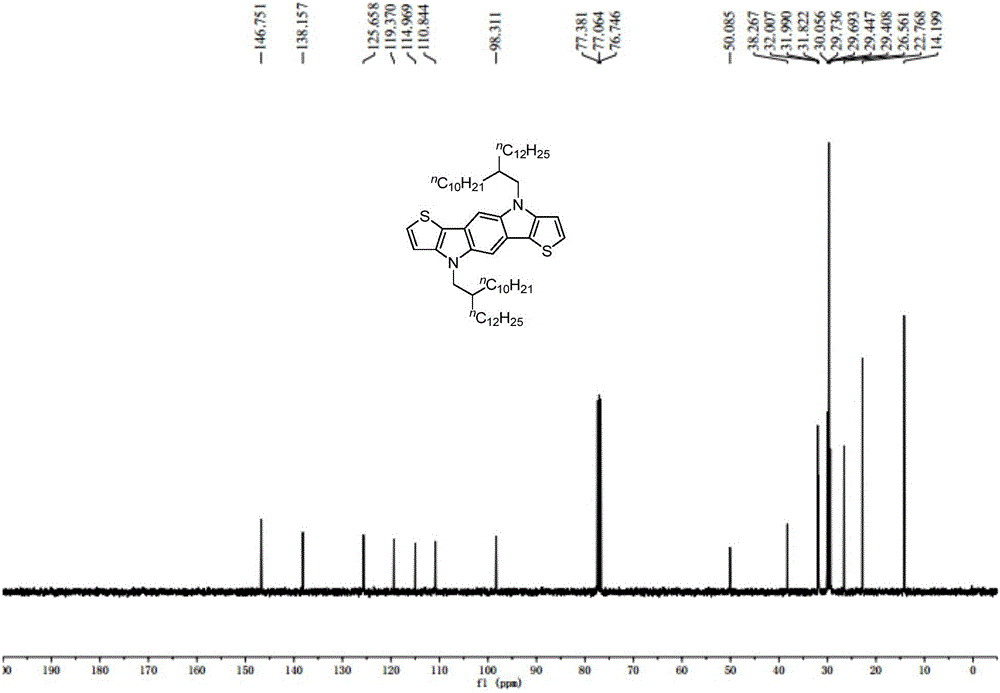
[0057] The preparation of embodiment 2 compound IVa
[0058]
[0059] Add compound IIV (279.0mg, 0.5mmol), sodium tert-butoxide (768.8mg, 8.0mmol), tris(dibenzylideneacetone) dipalladium (457.9mg, 0.5mmol), 2,2' - Bis-(diphenylphosphino)-1,1'-binaphthyl (BINAP, 1.25g, 2.0mmol) and toluene (10mL), after stirring at 25°C for 20 minutes, compound IIIV (707.4mg, 2.0mmol) was added , heated to 120°C for 24 hours. After cooling to room temperature, water (20 mL) was added, extracted with ether (30 mL×3), the combined organic phases were washed with saturated brine, dried over anhydrous magnesium sulfate, the solvent was removed by rotary evaporation, and the residue was separated by silica gel column chromatography (washing Compound IVa (white solid, 277.0 mg, yield: 80%) was obtained after removal of solvent: petroleum ether). 1 H NMR (400MHz, CDCl 3 )δ7.59(s,2H),7.34(d,J=5.2Hz,2H),7.05(d,J=5.2Hz,2H),4.16(d,J=7.2Hz,4H),2.17(m, 2H), 1.38–1.23(m, 80H), 0.88(m, 12H); 13 C NMR ...
Embodiment 3
[0060] The preparation of embodiment 3 compound 1b
[0061]
[0062] N-bromosuccinimide (NBS, 64.1mg, 0.36 mmol), kept at -78°C for 1 hour, extracted with ether (10mL×3), combined the organic phases and dried with anhydrous magnesium sulfate, and removed the organic solvent by rotary evaporation to obtain the crude product compound IIb, which was directly used in the following One step reaction.
[0063] At 0°C, under nitrogen protection, malononitrile (39.6 mg, 0.6 mmol), after the foam disappeared, the reaction was raised to room temperature for 30 minutes. The prepared malononitrile anion solution was transferred via cannula to a container containing compound IIb, tetrakis(triphenylphosphine)palladium (13.9 mg, 0.012 mmol) and 1,2-dimethoxyethane (5 mL). In a 50 mL three-necked flask, the reaction was heated under reflux for 3 hours under the protection of nitrogen. Then the reaction was cooled to room temperature and exposed to the air, dilute hydrochloric acid (10m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com