An all-solid-state lithium-air battery and its preparation method and application
An air battery and all-solid-state technology, which is applied in the direction of fuel cell half-cells and primary battery-type half-cells, can solve the problems of large battery polarization resistance, large battery contact resistance, and easy reaction with lithium metal anodes. , to achieve the effect of improving performance, increasing effective area, and large specific surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
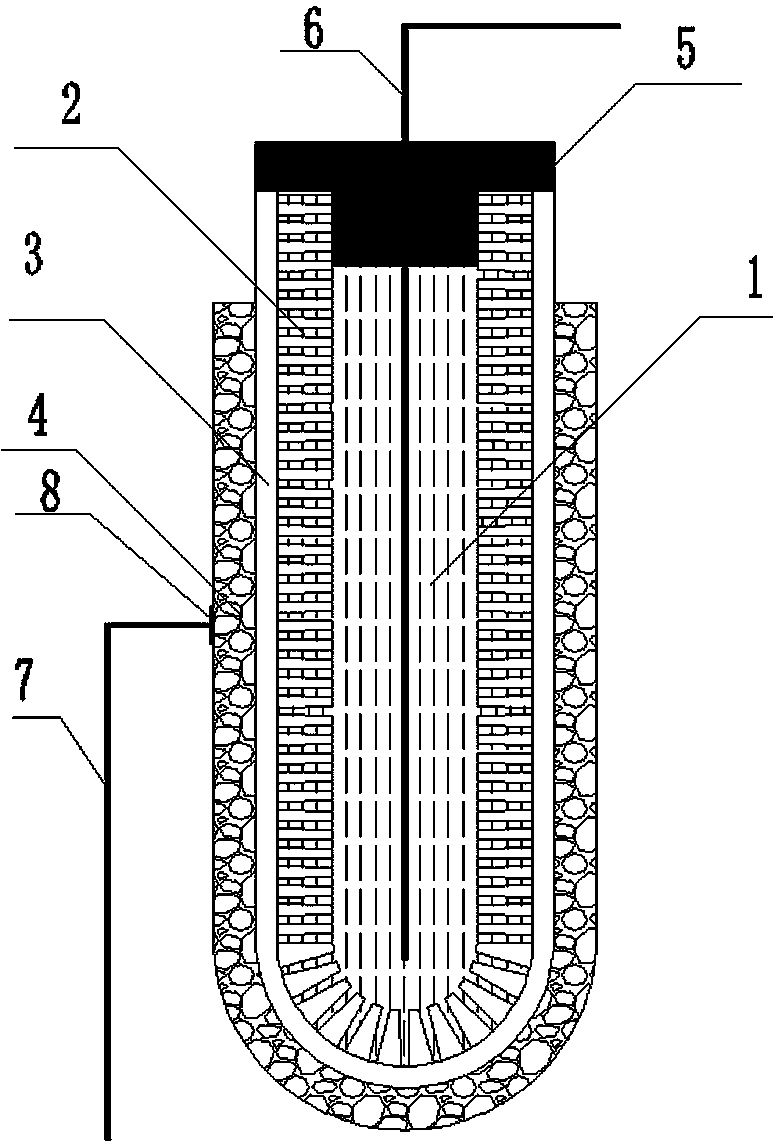
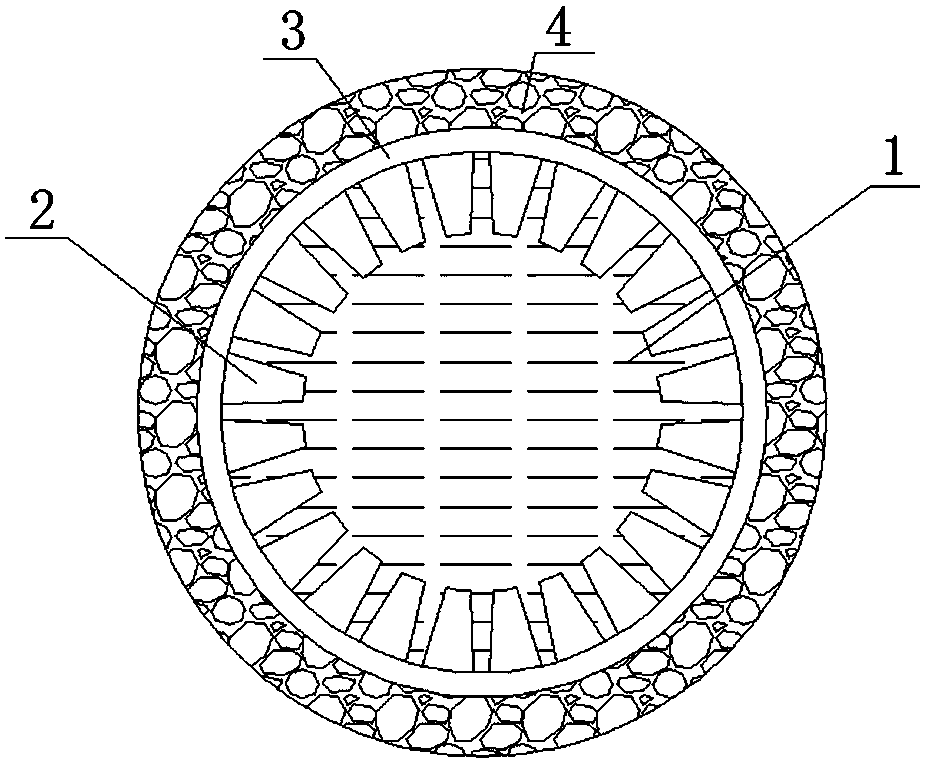
[0042] Accurately weigh 3.6 g Li 7 La 3 Zr 2 o 12 and 0.8 g polyethersulfone (PESf), then add 3.6 g N,N-dimethylpyrrolidone (NMP) and put it in an agate ball mill jar, and ball mill for 4h to obtain a uniform viscous slurry, which is transferred to a test tube, Use a glass rod with a diameter of about 0.3 cm to impregnate the slurry, pull it out, and rotate it at a constant speed. After the thickness is uniform, soak it in pure water quickly to cause a phase inversion process. After soaking for 20 minutes, take out the glass rod to obtain a closed end. The tubular green body was finally sintered at 1050 °C for 12 h at a heating rate of 1 °C / min to obtain a tubular porous ceramic support with one end closed. The support body is 4 cm long and 0.6 cm in outer diameter.
[0043] Accurately weighed 1.47 g Li 7 La 3 Zr 2 o 12 , 0.042 g triethanolamine, 0.054 g dibutyl phthalate, 0.054 g polyethylene glycol, 0.06 g polyvinyl butyral, and 4.32 g ethanol are placed in an agate ...
Embodiment 2
[0049] Accurately weighed 7.2 g Li 6.5 La 3 Zr 1.5 Ta 0.5 o 12 and 1.6 g polyethersulfone (PESf), then add 7.2 g N, N-dimethylpyrrolidone (NMP) and put it in an agate ball mill jar, and ball mill for 4 hours to obtain a uniform viscous slurry, which is transferred to a test tube, Use a glass rod with a diameter of about 0.8 cm to impregnate the slurry, pull it out, and rotate it at a constant speed. After the thickness is uniform, quickly soak it in pure water to undergo a phase inversion process. After soaking for 20 minutes, take out the glass rod to obtain a closed end. The tubular green body was finally sintered at 1050 °C for 16 h at a heating rate of 1 °C / min to obtain a tubular porous ceramic support with one end closed. The support body is 6 cm long and 1.2 cm in outer diameter.
[0050] Accurately weighed 1.47 g Li 6.5 La 3 Zr 1.5 Ta 0.5 o 12 , 0.042 g triethanolamine, 0.054 g dibutyl phthalate, 0.054 g polyethylene glycol, 0.06 g polyvinyl butyral, and 4.32...
Embodiment 3
[0056] Accurately weighed 7.2 g Li 6 La 3 Ta 1.5 Y 0.5 o 12 and 1.6 g polyethersulfone (PESf), then add 7.2 g N, N-dimethylpyrrolidone (NMP) and put it in an agate ball mill jar, and ball mill for 4 hours to obtain a uniform viscous slurry, which is transferred to a test tube, Use a glass rod with a diameter of about 0.6 cm to impregnate the slurry, pull it out, and rotate it at a constant speed. After the thickness is uniform, quickly soak it in pure water to undergo a phase inversion process. After soaking for 20 minutes, take out the glass rod to obtain a closed end. The tubular green body was finally sintered at 1050 °C for 24 h at a heating rate of 1 °C / min to obtain a tubular porous ceramic support with one end closed. The support body is 8 cm long and 1.0 cm in outer diameter.
[0057] Accurately weighed 1.47 g Li 6 La 3 Ta 1.5 Y 0.5 o 12 , 0.042 g triethanolamine, 0.054 g dibutyl phthalate, 0.054 g polyethylene glycol, 0.06 g polyvinyl butyral, and 4.32 g eth...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com