All-solid-state lithium-air battery and preparation method and application thereof
An air battery and all-solid-state technology, which is applied in the direction of fuel cell half-cells and primary battery-type half-cells, can solve the problems of large battery polarization resistance, large battery contact resistance, and increasing lithium ion transmission paths, etc., to achieve Effect of increasing effective area, improving performance, and large specific surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
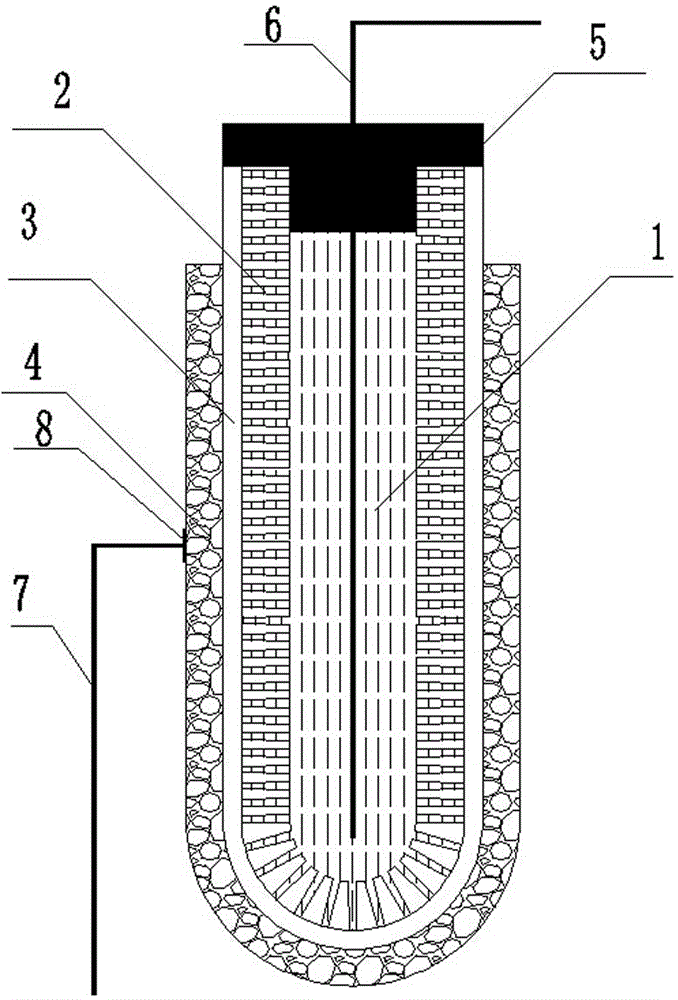
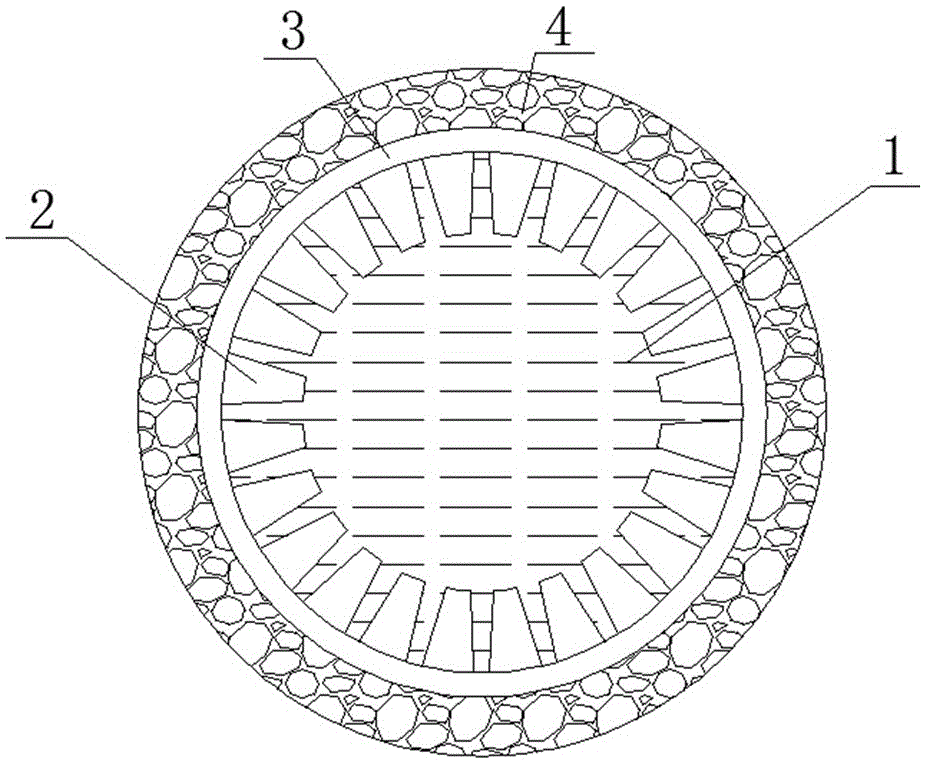
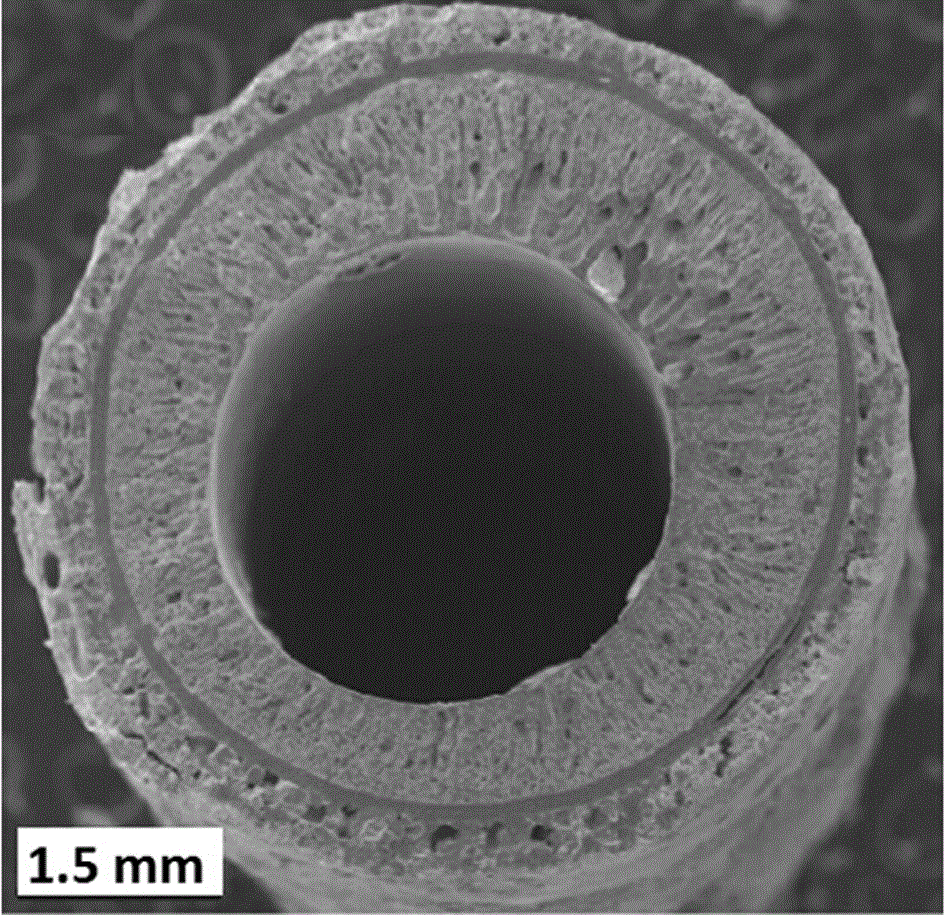
[0042] Accurately weigh 3.6gLi 7 La 3 Zr 2 o 12 And 0.8g polyethersulfone (PESf), add 3.6gN again, N-dimethylpyrrolidone (NMP) is placed in the agate ball mill jar, ball mills 4h, obtains uniform viscous slurry, slurry is transferred in the test tube, with A glass rod with a diameter of about 0.3 cm is impregnated with the slurry. After being pulled out, it is rotated at a constant speed. After the thickness is uniform, it is quickly immersed in pure water to undergo a phase inversion process. After soaking for 20 minutes, the glass rod is taken out to obtain a tube with one end closed. The green body was sintered at 1050°C for 12 hours at a heating rate of 1°C / min to obtain a tubular porous ceramic support with one end closed. The support body is 4 cm long and 0.6 cm in outer diameter.
[0043] Accurately weigh 1.47gLi 7 La 3 Zr 2 o 12 , 0.042g triethanolamine, 0.054g dibutyl phthalate, 0.054g polyethylene glycol, 0.06g polyvinyl butyral, and 4.32g ethanol were placed...
Embodiment 2
[0049] Accurately weigh 7.2gLi 6.5 La 3 Zr 1.5 Ta 0.5 o 12 And 1.6g polyethersulfone (PESf), add 7.2gN again, N-dimethylpyrrolidone (NMP) is placed in the agate ball mill jar, ball mills 4h, obtains uniform viscous slurry, slurry is transferred in the test tube, with A glass rod with a diameter of about 0.8 cm is impregnated with the slurry. After being pulled out, it is rotated at a constant speed. After the thickness is uniform, it is quickly immersed in pure water to undergo a phase inversion process. After soaking for 20 minutes, the glass rod is taken out to obtain a tube with one end closed. The green body was sintered at 1050°C for 16 hours at a heating rate of 1°C / min to obtain a tubular porous ceramic support with one end closed. The support body is 6 cm long and 1.2 cm in outer diameter.
[0050] Accurately weigh 1.47gLi 6.5 La 3 Zr 1.5 Ta 0.5 o 12 , 0.042g triethanolamine, 0.054g dibutyl phthalate, 0.054g polyethylene glycol, 0.06g polyvinyl butyral, and 4...
Embodiment 3
[0056] Accurately weigh 7.2gLi 6 La 3 Ta 1.5 Y 0.5 o 12 And 1.6g polyethersulfone (PESf), add 7.2gN again, N-dimethylpyrrolidone (NMP) is placed in the agate ball mill jar, ball mills 4h, obtains uniform viscous slurry, slurry is transferred in the test tube, with A glass rod with a diameter of about 0.6 cm is impregnated with the slurry. After being pulled out, it is rotated at a constant speed. After the thickness is uniform, it is quickly immersed in pure water to undergo a phase inversion process. After soaking for 20 minutes, the glass rod is taken out to obtain a tube with one end closed. The green body was sintered at 1050°C for 24 hours at a heating rate of 1°C / min to obtain a tubular porous ceramic support with one end closed. The support has a length of 8 cm and an outer diameter of 1.0 cm.
[0057] Accurately weigh 1.47gLi 6 La 3 Ta 1.5 Y 0.5 o 12 , 0.042g triethanolamine, 0.054g dibutyl phthalate, 0.054g polyethylene glycol, 0.06g polyvinyl butyral, and 4...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com