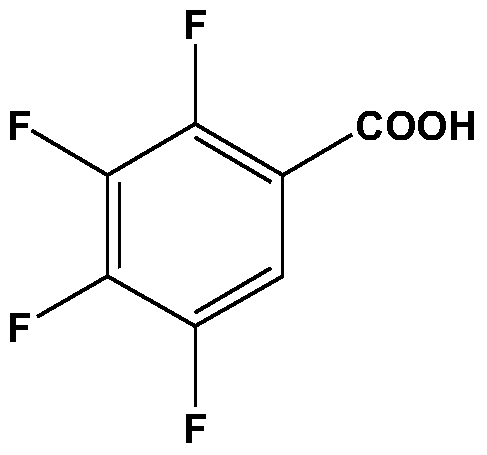
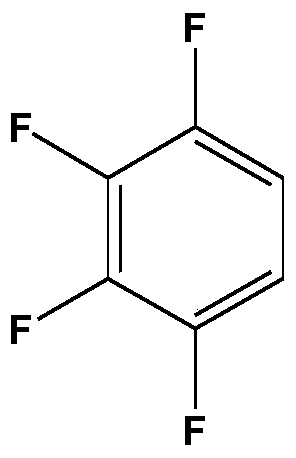
A method for preparing 2,3,4,5-tetrafluorobenzoic acid and 1,2,3,4-tetrafluorobenzene
A technology of tetrafluorobenzoic acid and tetrafluorophthalic acid, which is applied in chemical instruments and methods, preparation of halogenated hydrocarbons, organic chemistry, etc., can solve problems such as high toxicity and environmental pollution, and achieve improved alkalinity and reduced reaction Hazard, effect of promoting ionization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Add 300g of deionized water and 37.5g of tetrafluorophthalic acid into a 500mL intermittent high-pressure reactor, start stirring, and heat up to 230°C for decarboxylation for 150min; after the decarboxylation reaction is completed, open the exhaust valve to relieve pressure, cool to room temperature, and After the liquid-liquid separation, the organic phase and the aqueous phase are obtained; the aqueous phase is adjusted to pH 3-4, crystallized, and filtered to obtain crude 2,3,4,5-tetrafluorobenzoic acid; crude 2,3,4,5- Tetrafluorobenzoic acid was decolorized by active carbon, secondary crystallization, and vacuum-dried to obtain 15.4g 2,3,4,5-tetrafluorobenzoic acid product, and the product was analyzed by HPLC with a purity of 98.1% and a yield of 50.4%; After rectification, 5.2 g of 1,2,3,4-tetrafluorobenzene was obtained. The purity of the product analyzed by HPLC was 98.5%, and the yield was 22.0%.
Embodiment 2
[0032] Add 300g of ammonia solution with an ammonia concentration of 4g / L and 100.0g of tetrafluorophthalic acid into a 500mL intermittent high-pressure reactor, start stirring, and heat up to 230°C for 45 minutes for decarboxylation; after the decarboxylation reaction is completed, open the exhaust valve to relieve the pressure , recover the ammonia in the kettle; cool down to room temperature, and after standing still, the liquid-liquid layer is separated to obtain an organic phase and an aqueous phase; the aqueous phase is adjusted to a pH of 3 to 4, crystallized, and filtered to obtain crude 2,3,4,5-tetra Fluorobenzoic acid; crude 2,3,4,5-tetrafluorobenzoic acid was decolorized by activated carbon, secondary crystallized, and vacuum dried to obtain 19.3g of 2,3,4,5-tetrafluorobenzoic acid product, the product was analyzed by HPLC for purity 99.0% and a yield of 23.7%; the organic phase was rectified to obtain 43.2 g of 1,2,3,4-tetrafluorobenzene, and the product was analyze...
Embodiment 3
[0034] Add 300g of ammonia solution with an ammonia concentration of 2g / L and 60.0g of tetrafluorophthalic acid in a 500mL intermittent high-pressure reactor, start stirring, and heat up to 230°C for 60 minutes for decarboxylation; after the decarboxylation reaction is completed, open the exhaust valve to relieve pressure , recover the ammonia in the kettle; cool down to room temperature, and after standing still, the liquid-liquid layer is separated to obtain an organic phase and an aqueous phase; the aqueous phase is adjusted to a pH of 3 to 4, crystallized, and filtered to obtain crude 2,3,4,5-tetra Fluorobenzoic acid; crude 2,3,4,5-tetrafluorobenzoic acid was decolorized by activated carbon, secondary crystallized, and vacuum dried to obtain 13.0g of 2,3,4,5-tetrafluorobenzoic acid product, and the purity of the product was analyzed by HPLC 98.9% and a yield of 26.6%; the organic phase was rectified to obtain 25.8 g of 1,2,3,4-tetrafluorobenzene, and the product was analyze...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| flash point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com