In-vitro induction regulatory macrophage and preparation method and application
A macrophage and regulatory technology, applied in the medical field, can solve the problems of M1-type pro-inflammatory macrophage differentiation and instability, and achieve the effect of good therapeutic effect and stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] In this embodiment, the preparation method for inducing regulatory macrophages in vitro includes the following steps:
[0035] (1) Isolation and purification of rat bone marrow-derived macrophages
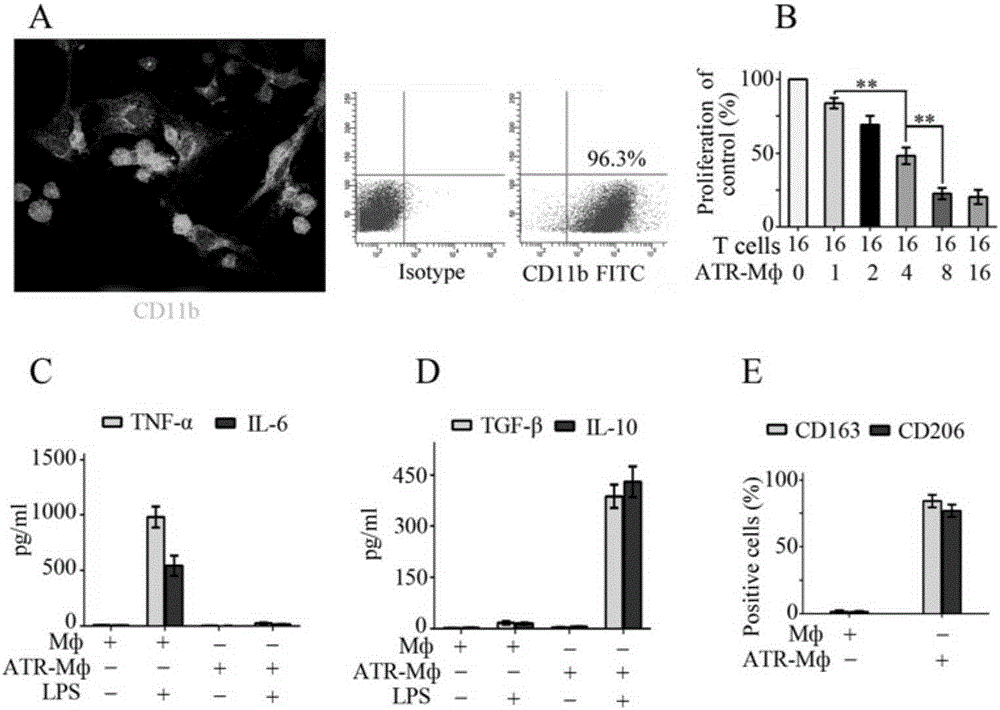
[0036] Rat bone marrow cells were collected with a medium containing 10% fetal bovine serum (Gibco, Grand Island, NY, USA), 100 U / ml penicillin, 100 μg / ml streptomycin, 2 mML-glutamine, 20 μM β-mercaptoethanol (the above reagents were purchased from Sigma, St.Louis, MO, USA) RPMI1640 (Gibco) culture medium. Recombinant rat macrophage colony-stimulating factor (M-CSF; PeproTech, Rocky Hill, NJ, USA, final concentration 50 ng / ml) was added to the medium to adjust the cell number to 2×10^6 / mL. Cultivate in 37℃, 5% CO2 incubator for 7 days, and change the medium every 2 days to obtain adherent cells.
[0037] (2) All-trans-retinoic acid (atRA) combined with tumor transforming factor-β (TGF-β)
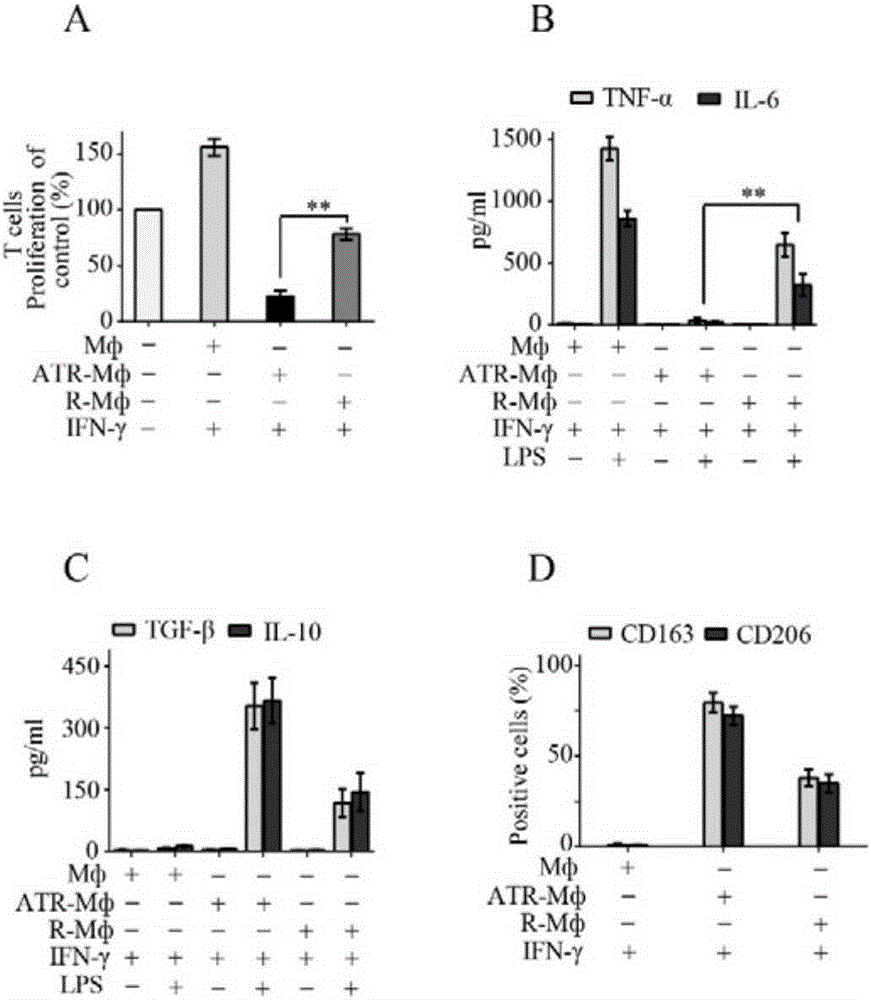
[0038] Induce the polarization of macrophages into regulatory macrophages (ATR-Mф)...
Embodiment 2
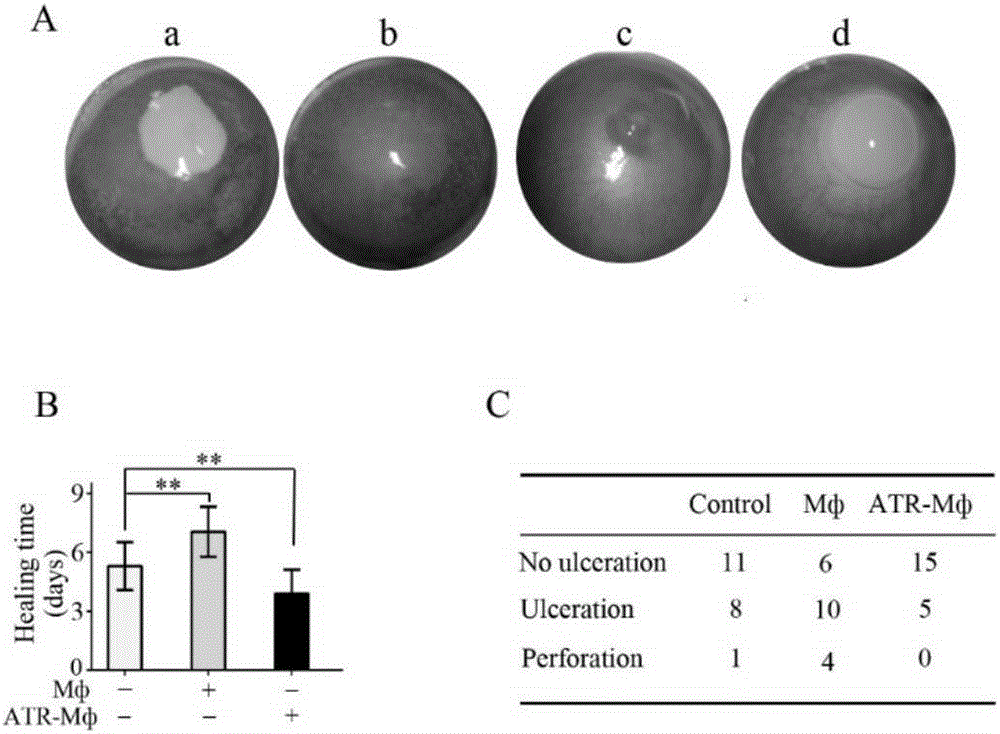
[0054] 1. Subconjunctival injection of ATR-Mф promotes healing of corneal alkali burn injury
[0055] A rat corneal alkali burn model was established according to our previous research method (InvestOphthalmolVisSci.2011; 52:9108–9115, PlosOne.2012; 7:e30842), and a Whatman filter paper piece with a diameter of 4 mm was cut out using a corneal trephine. After dripping 5 ul of 1M sodium hydroxide solution on each piece of filter paper with a micro-sampling gun, place the filter paper on the center of the cornea of the SD rat's right eye for 60 seconds, then rinse the cornea and conjunctival sac with 60ml of normal saline for 1 minute. After the model was established, check under the slit lamp every day (single-blind). The observation items mainly include: corneal epithelial defect, corneal ulcer, corneal perforation, corneal neovascularization and other related complications. The corneal epithelial defect after staining with 0.1% fluorescein was observed under the cobalt blu...
Embodiment 3
[0058] 2. Local subconjunctival injection of ATR-Mф significantly inhibited the occurrence of corneal transplant rejection in rats (see attached Figure 4 )
[0059] The allograft corneal rejection model was established according to the method of Thomas Ritter et al. (InvestOphthalmolVisSci, 2007; 48:1043-1052), Wistar rats were used as transplant donors, SD rats were used as recipients, the diameter of the graft was 3.5 mm, and the diameter of the implant bed was 3.0 mm , 10 / 0 nylon suture, interrupted suture 8 stitches.
[0060] The corneal transparency, edema, and neovascularization were checked under slit lamp every day after operation, and the scores and photos were taken to determine the time of rejection. According to the internationally recognized Holland's criteria (Cornea.1991; 10(5):374-80), the rejection criteria for corneal transplantation are established. When the sum of the scores of corneal graft transparency, edema, and new blood vessels exceeds 6 points, it ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com