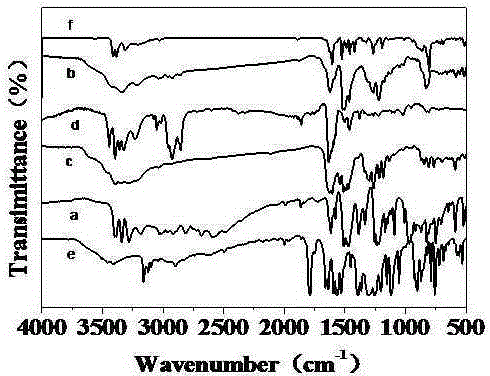
Diamine monomer containing carbazole structure and having high planarity, synthetic method and application thereof
A technology of diamine monomer and synthesis method, applied in the field of material science, to achieve the effects of easy purification, strong interaction force and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Synthesis of N1,N1'-(9-methyl-9H-carbazole-3,6-diyl)bis(benzene-1,4-diamine):
[0071] Synthetic intermediate 3,6-dibromo-9-methyl-9H-carbazole:
[0072] Pour 3.25g (0.01mol) of 2,7-dibromocarbazole (3,6-dibromo-9H-carbazole), 150ml of DMF, and 1.5g of NaH suspension into a 250ml three-necked flask, and add 4ml of iodomethane successively, Methyl iodide was added for more than 10 minutes, protected by argon, and refluxed for 18 hours. The reaction solution was poured into ice water, a large amount of precipitation was precipitated, filtered with suction, washed with water, and a white solid was obtained. It was dried in vacuum at 80°C for 24 hours, and the yield was about 90%. The intermediate structure is as follows:
[0073]
[0074] Synthetic intermediate 9-methyl-9H-carbazole-3,6-diamine:
[0075] Add 3.39g (0.01mol) of 3,6-dibromo-9-methyl-9H-carbazole, appropriate amount of cuprous oxide, 50ml of NMP, 13ml of ammonia water (29%, 0.2mol) into a 200ml pressure ...
Embodiment 2
[0083] Synthesis of N3,N6-bis(4-aminophenyl)-9H-carbazole-3,6-dicarboxamide:
[0084]
[0085] Synthetic intermediate 9H-carbazole-3,6-dicarbonitrile:
[0086] Add 3.25g (0.01mol) 3,6-dibromo-9H-carbazole, 2.762g (0.05mol) cyanocopper, and 50ml of dry NMP into a 500ml three-necked flask, reflux at 140°C for 24h, then add water (180mL), HCl ( 60 mL) and FeCl3 (4.19 g, 25.8 mmol) were poured into the reaction solution and stirred for 1 h, cooled to room temperature, filtered to obtain a brown precipitate, and washed with water, the obtained solid was redissolved in dichloromethane and washed with water, and the solvent was removed under reduced pressure to obtain a crude product It was a brown solid, which was triturated with methanol to give the desired product 9H-carbazole-3,6-dicarbonitrile as an off-white solid 0.65 g, 31% yield. The intermediate structure is as follows:
[0087]
[0088] Synthetic intermediate 9H-carbazole-3,6-dicarboxylic acid:
[0089] Add 2.17g ...
Embodiment 3
[0100] Synthesis of 4,4'-((9H-carbazole-3,6-diyl)bis(oxy))dianiline:
[0101]
[0102] Synthesis of intermediate 3,6-bis(4-nitrophenoxy)-9H-carbazole:
[0103] Add 3.250g (0.01mol) 2,7-dichloro-9H-carbazole and 3.478g (0.025mol) 4-nitrophenol into a 500ml three-necked flask, use DMF as a solvent, add an appropriate amount of NaOH, heat the oil bath to 110°C and add An appropriate amount of CuI was refluxed for 22 hours, and the reaction solution was poured into water, and a large amount of precipitates precipitated out. Filter with a funnel, evaporate the solvent under reduced pressure. The product was purified by column chromatography using silica gel as the stationary phase. The product was collected and spin-dried, and dried in vacuum at 80°C for 24 hours to obtain 2.5 g of 3,6-bis(4-nitrophenoxy)-9H-carbazole as a light yellow solid. The yield was was 71.5%. The intermediate structure is as follows:
[0104]
[0105] Synthesis of 4,4'-((9H-carbazole-3,6-diyl)bis(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com