A kind of purification method of recombinant human epidermal growth factor
A technology of epidermal growth factor and purification method, applied in the field of purification of recombinant human epidermal growth factor (rhEGF), can solve the problems of difficulty in preparation, large differences, influence on activity and safety, etc. The effect of few process steps and high practical value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1. Purification of rhEGF protein
[0034] Collect the culture supernatant (1000 mL) containing rhEGF protein, adjust the pH to 8.0 with 1mol / L NaOH solution, centrifuge to remove insoluble impurities, and collect the supernatant.
[0035] Take the Ni Sepharose 6 Fast Flow affinity chromatography medium, put it into the normal pressure chromatography column, and use the equilibrium buffer (25mmol / L PB buffer, pH8.0) at a flow rate of 150cm / h (the loading and elution speed and the equilibrium The speed is the same, the same below) After equilibrating for 3 column volumes, load the centrifuged supernatant containing rhEGF, re-equilibrate with equilibration buffer (25mmol / L PB buffer, pH8.0), and detect A 280nm , the breakthrough peak was discarded, and when A 280nm After falling to the baseline, elute with equilibration buffer (25mmol / LPB buffer, pH8.0) containing 20mM imidazole, and collect the elution peak containing rhEGF protein;
[0036] Take Source 15RPC re...
Embodiment 2
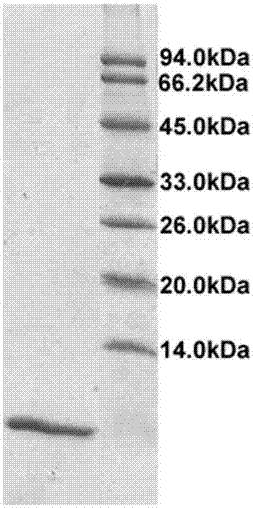
[0038] Example 2. Determination of the purity of rhEGF protein SDS-PAGE
[0039] (1) 15% separation gel configuration
[0040] Double distilled water: 3.3 mL; 1.5 mol / L Tris-HCl (pH 8.8): 2.5 mL; 10% SDS: 100 μL; 30% acrylamide monomer stock solution: 4.0 mL; TEMED: 4 μL; 10% persulfuric acid Ammonium: 100 μL; total volume: 10 mL. After mixing, put it into the gap between the glass plates of the electrophoresis tank, and add about 1cm of double distilled water on the surface of the gel. After the gel naturally coagulates, remove the double distilled water and put a comb in the gap.
[0041] (2) 5% stacking gel configuration
[0042] Double distilled water: 3.4 mL; 1mol / L Tris-HCl (pH6.8): 0.63 mL; 10% SDS: 50 μL; 30% acrylamide monomer stock solution: 0.83 mL; TEMED: 5 μL; 10% persulfate Ammonium: 150 μL; total volume: 5 mL. After mixing, add to the crevice and do not pass through the comb hole. After condensation, carefully pull out the comb, and rinse the sample hole wit...
Embodiment 3
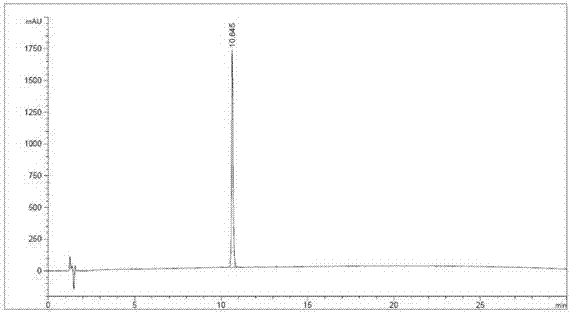
[0053] Embodiment 3. rhEGF RP-HPLC purity determination
[0054] Instrument: Aglient 1100 HPLC; Chromatographic column: Welch Ultimate XB-C18 (5μm 4.6×100mm); Mobile phase: Liquid A is 0.1% trifluoroacetic acid + aqueous solution; Liquid B is 0.1% trifluoroacetic acid + acetonitrile solution; flow rate: 1.0 mL / min; Elution mode: 0-30min, B from 5-95%; Detection wavelength: 220nm. Take 10 μL of rhEGF sample, and perform full gradient RP-HPLC purity determination according to the above conditions.
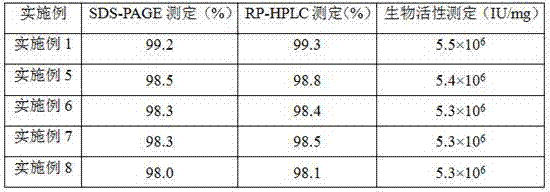
[0055] as attached figure 2 As shown, there is no obvious impurity band in the RP-HPLC spectrum of the rhEGF sample, and the RP-HPLC purity of the main peak is 99.3%, which is higher than the high-performance liquid chromatography stipulated in the Chinese Pharmacopoeia "the main peak area of human epidermal growth factor should not be less than the total area 95.0%" standard.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com