Azo hydrophilic compound as well as preparation method and application thereof
A hydrophilic compound and azo technology, applied in the preparation of sulfonic acid, organic chemistry, etc., can solve the problems that affect the cis-trans isomerization efficiency of azo groups, poor migration resistance of colored materials, and difficulty in reaching 100%. Achieve the effects of long-lasting maintenance of azo functional properties, not easy migration, and simplified production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
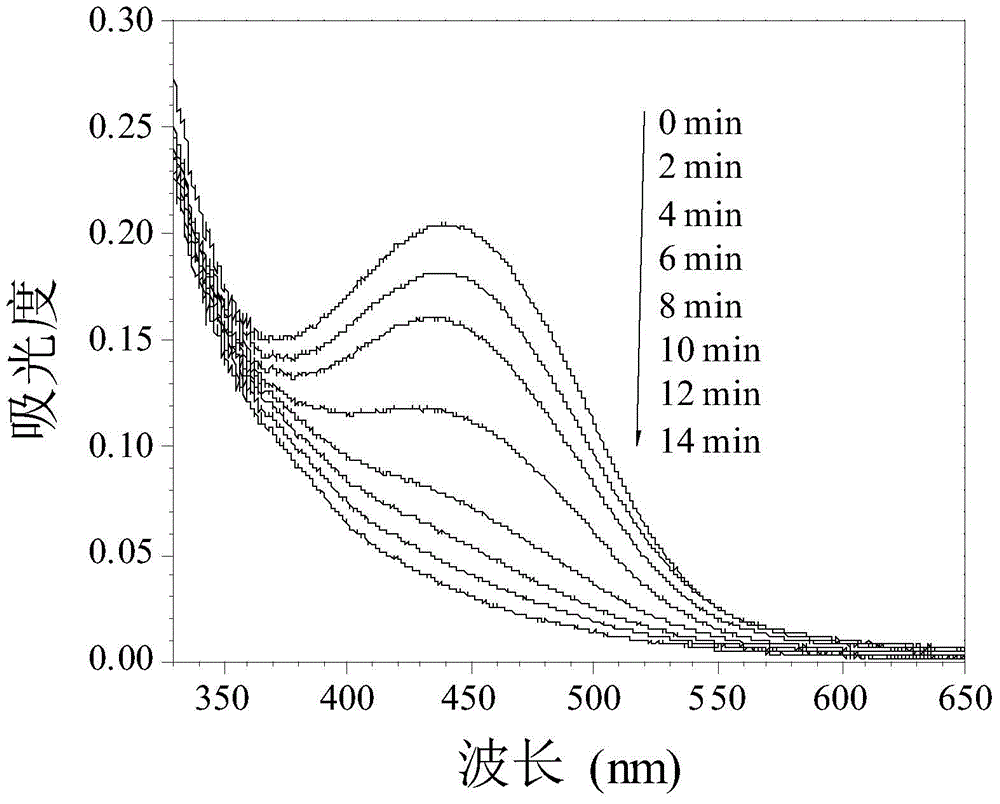
Examples
Embodiment 1
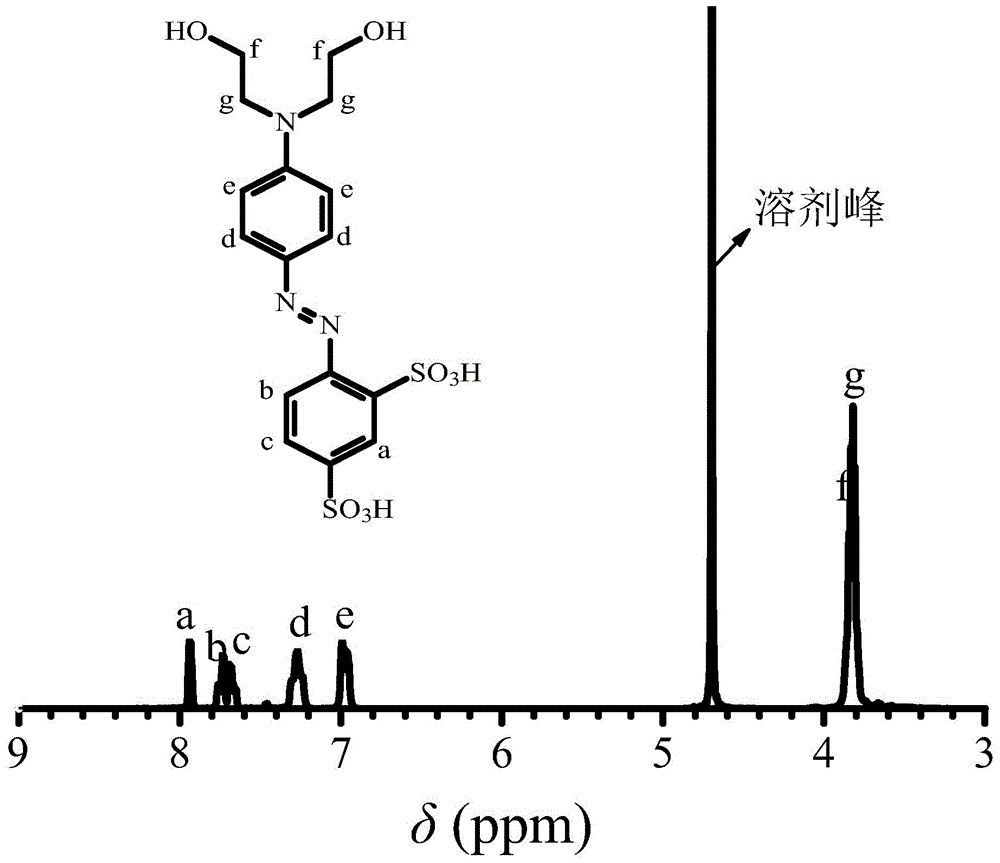
[0031] 1. Take 15 grams of aniline-2,4-disulfonic acid and add it to a 250 ml three-necked bottle, add 60 ml of water at room temperature to fully dissolve it, slowly add 17.8 grams of concentrated hydrochloric acid (mass concentration 36.5%, the concentration used below The same as hydrochloric acid) and put it in an ice-water bath, keep the reaction system at 0-5°C, add dropwise 4.1 grams of sodium nitrite with a concentration of 50% aqueous solution, keep the ice-water bath for 1 hour to obtain aniline-2,4-disulfonic acid Diazonium salt solution;
[0032] 2. Dissolve 11.2 g of N,N-dihydroxyethylaniline in 35 ml of methanol and 0.5 g of acetic acid to form a N,N-dihydroxyethylaniline solution, add it to a 1000 mL three-necked flask, and place the three-necked flask in an ice-water bath , keep the reaction system at 0-5°C, drop the diazonium salt solution of aniline-2,4-disulfonic acid prepared in step 1 into the N,N-dihydroxyethylaniline solution, and drop it within 0.5h. K...
Embodiment 2
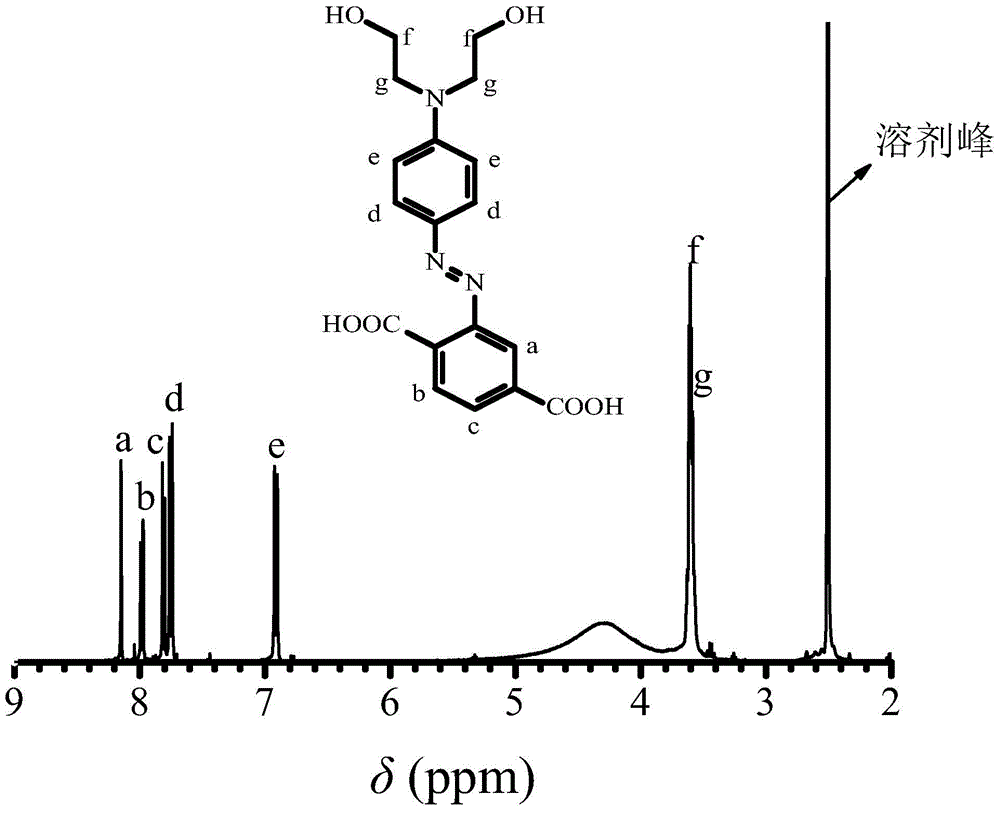
[0035] 1. Take 5 grams of 2-aminoterephthalic acid and add it to a 150 ml three-necked bottle, add 50 ml of water at room temperature to fully dissolve it, slowly add 9 grams of concentrated hydrochloric acid and place it in an ice-water bath to keep the reaction system 0- 5°C, dropwise add 1.89 grams of sodium nitrite in an aqueous solution with a concentration of 50% by mass, and keep the reaction in an ice-water bath for 1 hour to obtain a diazonium salt solution of 2-aminoterephthalic acid;
[0036] 2. Dissolve 5.11 grams of N,N-dihydroxyethylaniline in 10 ml of methanol and 0.5 grams of acetic acid to form a N,N-dihydroxyethylaniline solution, add it to a 500ml three-necked flask, and place the three-necked flask in an ice-water bath , keep the reaction system at 0-5°C, drop the diazonium salt solution of aniline-2,4-disulfonic acid prepared in step 1 into the N,N-dihydroxyethylaniline solution, and drop it within 0.5h. Keep the pH of the reaction system at 6 with 1 mol / L...
Embodiment 3
[0039] 1. Take 300 grams of aniline-2,4-disulfonic acid and add it to the reaction kettle, add 1 liter of water at room temperature to fully dissolve it, slowly add 356 grams of concentrated hydrochloric acid and place it in an ice-water bath to keep the reaction system 0-5 ℃, quickly drop 82 grams of sodium nitrite in an aqueous solution with a concentration of 50% by mass, and keep the reaction in an ice-water bath for 1 hour to obtain a diazonium salt solution of aniline-2,4-disulfonic acid;
[0040] 2. Dissolve 222 grams of N,N-dihydroxyethylaniline in 600 ml of methanol and 10 grams of acetic acid to form a N,N-dihydroxyethylaniline solution, add it to the reaction kettle, and place the three-necked bottle in an ice-water bath , keep the reaction system at 0-5°C, drop the diazonium salt solution of aniline-2,4-disulfonic acid prepared in step 1 into the N,N-dihydroxyethylaniline solution, drop it within 0.5h, pass 1mol / L sodium hydroxide aqueous solution to keep the pH o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com