Anti-hepatitis b virus X protein polypeptide drug
A hepatitis B virus and drug technology, applied in the direction of antiviral agents, antineoplastic drugs, drug combinations, etc., to achieve good stability, obvious inhibitory effect, and significant pharmaceutical effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] Example 1: Design and preparation of polypeptides
[0089] Synthetic polypeptide functional fragment D-TTK001:
[0090] In the present invention, the amino acid sequence synthesized by artificial synthesis is Gly-D-Ser-D-Ala-D-Val-D-Met-D-Phe-D-Ser-D-Ser-D-Lys-D-Glu- D-type polypeptide of D-Arg-Gly (SEQ ID NO: 1) (hereinafter referred to as D-TTK001). The preparation of the polypeptide adopts a solid-phase synthesis method, such as using an AAPPTECAPex396 polypeptide synthesis instrument (purchased from Hong Kong Universal Analytical and Testing Instrument Co., Ltd.), in a closed explosion-proof glass reactor to make amino acids according to the sequence shown in SEQ ID NO: 1, From C-terminus-carboxyl-terminus to N-terminus-amino-terminus, this refers to the first amino acid sequence added to the amino acid sequence Gly-D-Ser-D-Ala-D-Val-D-Met-D-Phe-D-Ser The amino acid monomer of -D-Ser-D-Lys-D-Glu-D-Arg-Gly is the C-terminal Gly, then D-Arg, then D-Glu, until the la...
Embodiment 2
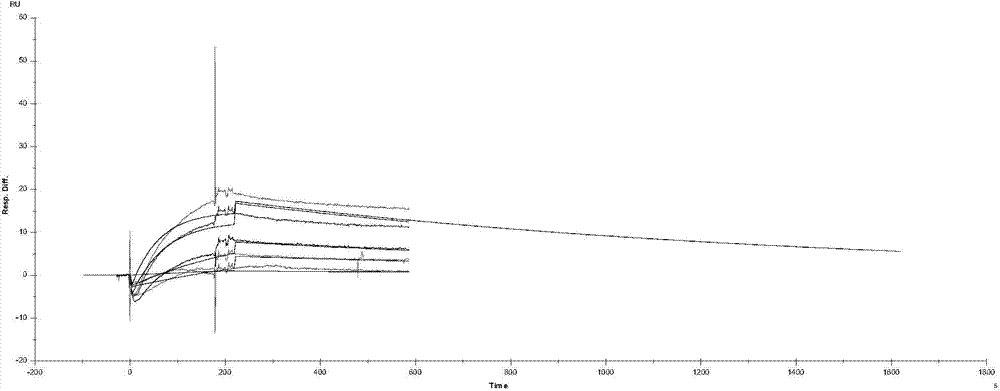
[0093] A. In vitro binding assay
[0094]The plasmid (pET-30a-HBx) for recombinantly expressing the HBx gene was constructed and preserved by itself (Zhang H, et al. J Biomed Biotechnol doi: 10.1155 / 2009 / 289068), and the method in this document was used to complete the expression and purification of HBx, For in vitro binding assays. Biacore3000 biomacromolecule interaction analyzer (manufactured by GE Healthcare) was used to determine the in vitro binding force between polypeptide D-TTK001 and recombinantly expressed HBx protein. Different concentrations of peptides were injected at a rate of 30 μl / min for 180 seconds. During the dissociation phase, HBS-EP buffer was injected at a rate of 30 μl / min for 900 seconds, followed by injection of 2 needles of 1 mM NaOH at a flow rate of 30 μl / min for 20 seconds to regenerate the chip. All signals were corrected via channel 1 as a control channel. The determination of the binding force was tested twice. In the first test, HBX was i...
Embodiment 3
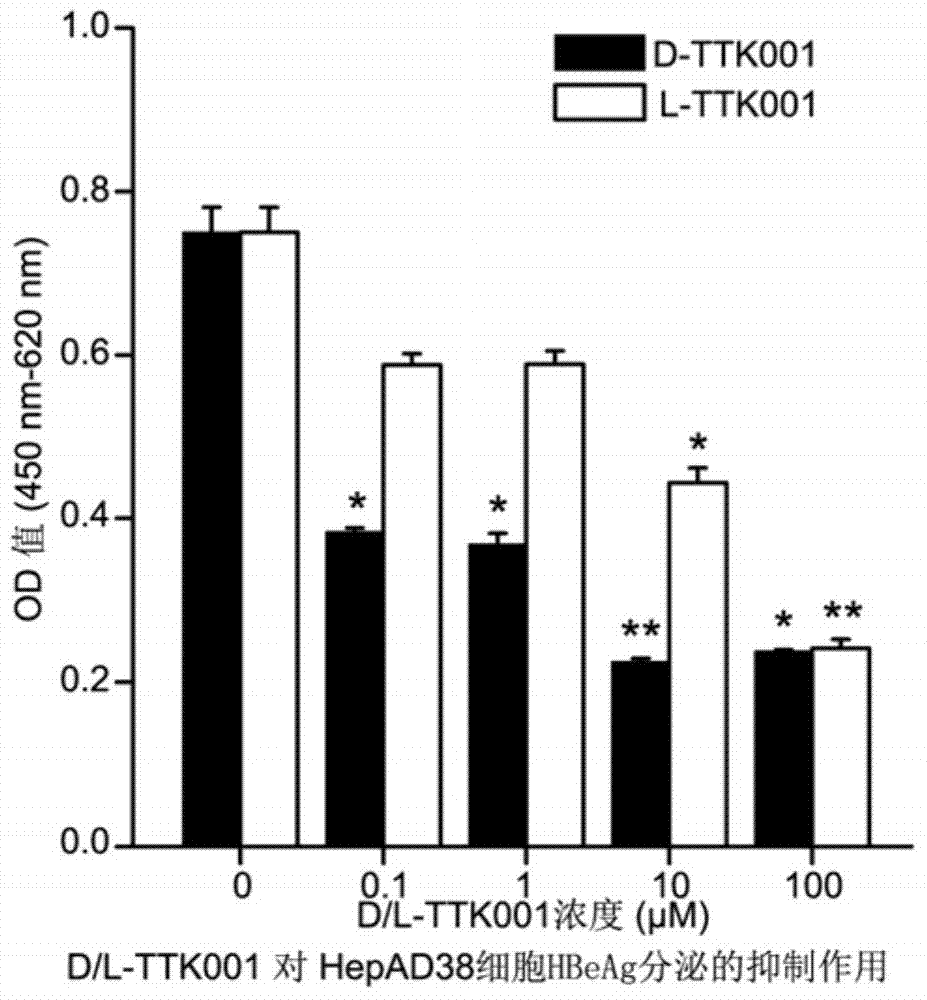
[0129] Example 3: Experiments on the effectiveness of polypeptides in vivo
[0130] 1. Effect of D-TTK001 on HBV DNA in HBV transgenic mice
[0131] (1) The source and characteristics of HBV transgenic mice Guangzhou Junke Taite Pharmaceutical Technology Co., Ltd.
[0132] HBV transgenic mice were purchased from Guangzhou Junke Taite Pharmaceutical Technology Co., Ltd. The animal model is a transgenic mouse Tg(HBV1.3genome)Swb successfully established and bred by Guangzhou Junke Taite Pharmaceutical Technology Co., Ltd. by microinjection method with high expression replication type 1.3 copies of HBV whole gene. Serum HBsAg and HBeAg can be detected with conventional ELISA kits; 93.93% of positive transgenic mice serum HBV DNA has reached 104-106 copy / ml; liver tissue immunohistochemistry has the expression of HBsAg (cytoplasmic type) and HBcAg (nuclear type). Gender, different ages, and different times of the day had no significant effect on the expression of HBsAg, that is,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com