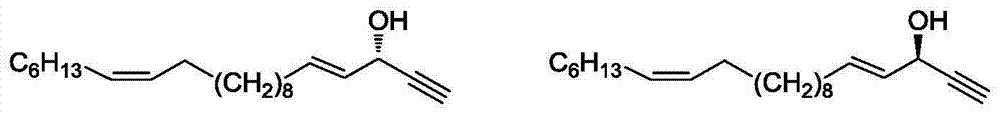
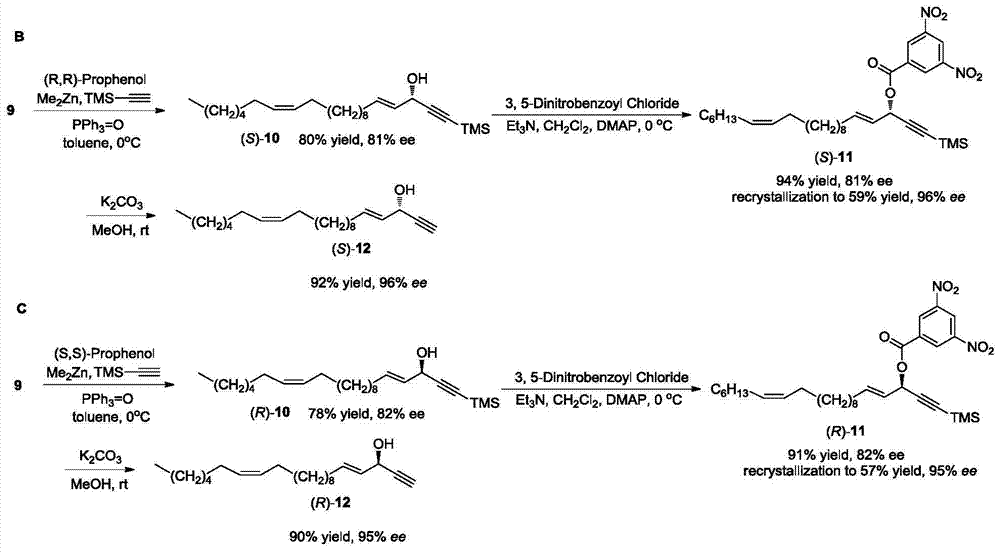
Marine natural product (+)-(4E, 15Z)-4, 15-docosadienoic-1- alkyne-3-alcohol and synthetic method of enantiomer thereof
A technology for natural products and synthetic methods, applied in asymmetric synthesis, organic chemistry methods, chemical instruments and methods, etc., can solve the problems of low yield, harsh reaction conditions, poor practicability, etc., and achieve high optical purity of products and simple steps. , the effect of high total yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
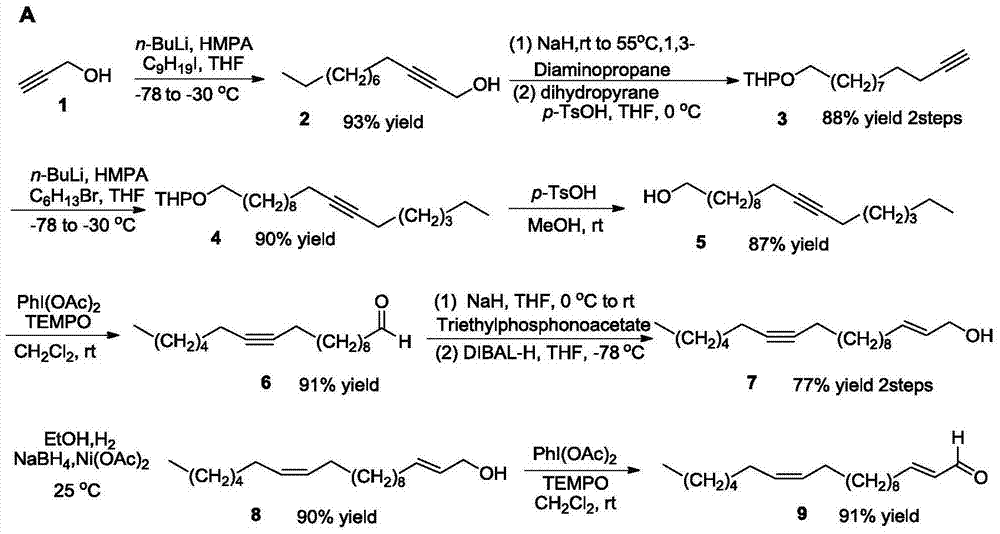
[0023] Embodiment 1 prepares 2-dodecyn-1-alcohol
[0024] Under nitrogen protection, add anhydrous THF (50 mL), freshly distilled propynyl alcohol (3.36 g, 60 mmol) and hexamethylphosphoric triamide (42 mL, 240 mmol) into a 250 mL Shrek tube, and stir well. The mixture was cooled to -78°C, and n-butyllithium solution (48 mL, 2.5 mol / L in hexane, 120 mol) was slowly added dropwise. After the dropwise addition, the mixture was stirred at -78°C for 30min, then warmed up to -30°C, and stirred for 3h. Iodo-n-nonane (16.8 g, 66 mmol) was slowly added to the mixture, and the stirring reaction was continued for 12 h, and the reaction liquid was naturally warmed to room temperature. After the reaction, add saturated NH 4 Aqueous Cl (5 mL) was used to quench the reaction. The aqueous phase was extracted with ether (40mL×3), the organic phases were combined, anhydrous Na 2 SO 4 After drying, the solvent was removed under reduced pressure to obtain a crude product. Finally, it was p...
Embodiment 2
[0025] Example 2 Preparation of 2-(11-dodecynyloxy)tetrahydropyran
[0026] Under nitrogen protection, in a 500mL four-neck flask equipped with mechanical stirring, a thermometer and a reflux condenser, add 1,3-propanediamine (150mL), add NaH (4.6g, 60% in mineral oil, 115mmol), the mixture was heated to 70°C, and stirring was continued for 1h after a large number of bubbles emerged. Then the mixture was cooled to room temperature, 2-dodecyn-1-ol (5.0 g, 27.5 mmol) was slowly added dropwise, the reaction solution was heated to raise the temperature to 55°C, and the reaction was continued to stir for 12 h. After the reaction was complete, a small amount of ice water (3 mL) was added to quench the reaction. The organic phase was extracted with ether (50mL×3), the organic phases were combined, anhydrous Na 2 SO 4 After drying and concentrating under reduced pressure, a colorless viscous liquid terminal acetylenic alcohol crude product was obtained, which could be directly used...
Embodiment 3
[0028] Embodiment 3 prepares 2-(11-octadecynyloxy group) tetrahydropyran
[0029] Under nitrogen protection, add anhydrous THF (30mL), 2-(11-dodecynyloxy)tetrahydropyran (1.3g, 5mmol) and hexamethylphosphoric triamide (3.5mL) into a 100mL Shrek bottle mL, 20mol), stir well. The mixture was cooled to -78°C, and n-butyllithium (4 mL, 2.5mol / L in hexane, 10 mmol) was slowly added dropwise. After the addition was complete, the mixture was stirred at -78°C for 30 min. Then the temperature was raised to -30°C and stirring was continued for 3h. Then bromo-n-hexane (0.9 g, 5.5 mmol) was added slowly, and the stirring reaction was continued for 12 h, and the reaction liquid was naturally warmed to room temperature. After the reaction, add saturated NH 4 Aqueous Cl solution (1 mL) quenched the reaction. The aqueous phase was extracted with ether (40mL×3), the organic phases were combined, anhydrous Na 2 SO 4 After drying, the solvent was removed under reduced pressure to obtain a ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com