Alpha-amylase Amy16, as well as gene and application thereof
An amylase and gene technology, which is applied to alpha-amylase Amy16 and its gene and application fields, can solve the problem that the enzymatic properties of amylase cannot be fully satisfied, achieve good thermal stability and alkali tolerance, and reduce stability. , the effect of strong stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1 The Extraction of Pacific Flameovirga Pacifica H2 (Flammeovirga Pacifica H2) Genomic DNA
[0042] Use an inoculation loop to pick the strains preserved at -80°C, and mark them on a 2216E medium plate (a bacterial culture medium formulated with 1% tryptone, 0.2% yeast extract, 1.5% agar powder, and 100 mL of seawater). Line isolation of single colonies. The streaked plate was cultured at 37°C overnight, and a single colony was picked and inoculated into 5 mL of 2216E liquid medium, cultured overnight at 37°C with shaking, collected by centrifugation at 6000 g for 8 min, and the pellet was resuspended in 567 μL TE buffer (10 mM Tris- HCl, 1mM EDTA, pH 8.0), add 30μL 10% SDS and shake well, then add 3μL proteinase K (20mg / mL), mix gently, and bathe in 37℃ water for 1h. Add 100 μL 5mol / L NaCl, mix thoroughly, then add 80 μL CTAB (cetyltriethylammonium bromide) / NaCl, mix gently, and bathe in water at 65°C for 10 minutes. Extract twice with phenol / chloroform / isoam...
Embodiment 2
[0043] The PCR amplification of embodiment 2α-amylase gene amy16
[0044] According to the Flammeovirga Pacifica H2 genome sequencing and gene annotation results, the primers were designed as follows:
[0045] F: 5′-CG GAATTC ATGGAGGACAATGAG AGT-3' (the line is the EcoR I restriction site) (SEQ ID NO.3);
[0046] R: 5′-GCG AAGCTT TTATTTCAATGTCCAAATAG-3' (HindIII restriction site is underlined) (SEQ ID NO.4).
[0047] Using the extracted Flammeovirga Pacifica H2 genomic DNA as a template, the full-length amy16 gene was amplified with primers F and R. The PCR reaction conditions are as follows: 50 μL reaction system contains: 10×PCR buffer 5 μL, dNTP 5 μL, bidirectional primer with a final concentration of 10 pmol, PrimeStar DNA polymerase 1 μL, template DNA 1 μL, make up the volume to 50 μL with double distilled water; Reaction conditions: 94°C for 5min; 95°C for 90s, 58°C for 90s, 72°C for 90min (30cycles); 72°C for 10min. The purified PCR product was digested, and then...
Embodiment 3
[0048] Expression and purification of embodiment 3 recombinant Amy16
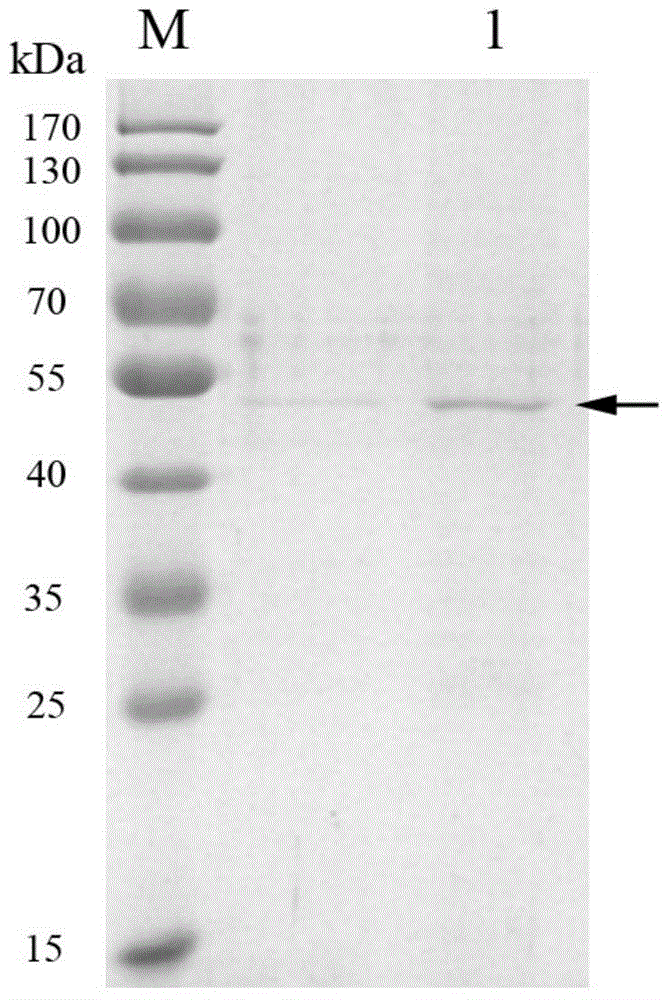
[0049] The recombinant vector pET-His-amyl6 obtained in the above-mentioned Example 2 was transformed into E. coli BL21, and positive clones were selected in LB medium containing 100 μg / mL ampicillin (a bacterial culture medium, formulated as 1% tryptone, 0.5% yeast extract, 1% NaCl) at 37 ° C shake culture to OD 600 = 0.6, add isopropylthio-β-D-galactoside (IPTG) to a final concentration of 1 mM, and after induction at 15°C for 6 hours, collect the bacterial liquid into a 50 mL centrifuge tube, and centrifuge at 12,000 g to precipitate bacterial cells. Bacterial cells were resuspended in 2.5mL of Tris / HCl (pH8.0) buffer, ultrasonically disrupted thalli, centrifuged at 12000g for 15min, and the supernatant was collected. The purification process followed the nickel ion affinity chromatography column purification kit (purchased from Qiagen Company) instructions. The purified protein was analyzed by 10% SDS...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com