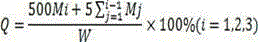Controlled release tinidazole ointment suitable for being orally administered
A technology of tinidazole and ointment, applied in the field of sustained and controlled release tinidazole ointment, which can solve the problems of cumbersome nano-liposome preparation process, foreign body sensation on periodontal strips, and difficult removal, etc., and achieve long-term maintenance Effects of topical administration, relieving disease, and improving drug release performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
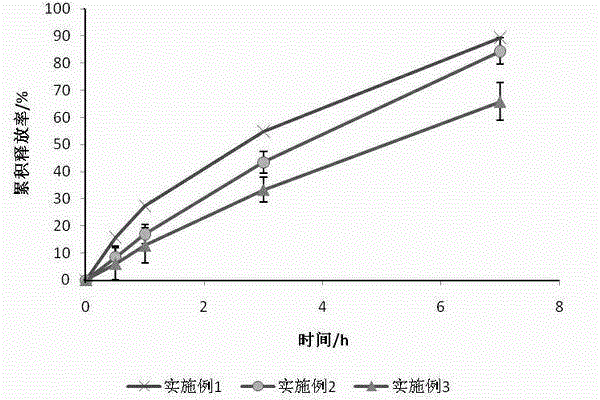
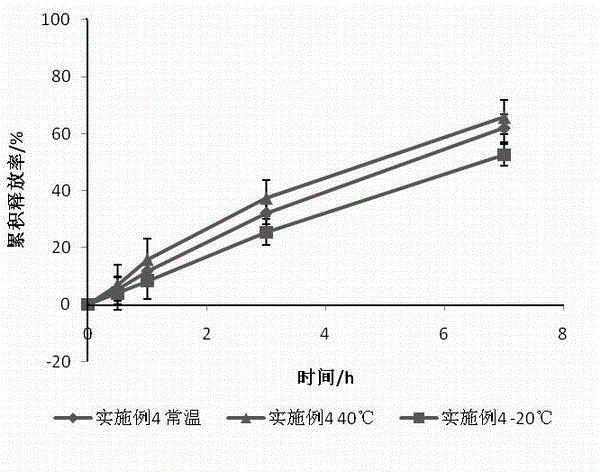
Embodiment 1
[0032] Put 70g of petrolatum and 7g of glyceryl behenate in a beaker and stir evenly, heat the above matrix to a viscous fluid state, add 5g of sucrose fatty acid ester (HLB=15) and 8g of hydroxypropyl methylcellulose in turn and stir uniformly, add 10 g of ground tinidazole and stir evenly, then heat the above mixture to a viscous fluid state and knead to obtain an ointment.
Embodiment 2
[0034] Put 75g of petrolatum and 5g of glyceryl behenate in a beaker and stir evenly, heat the above matrix to a viscous fluid state, add 6g of sucrose fatty acid ester (HLB=15) and 10g of hydroxypropyl methylcellulose to it and stir uniformly, add 4 g of ground tinidazole and stir evenly, then heat the above mixture to a viscous fluid state and knead to obtain an ointment.
Embodiment 3
[0036] Put 85g of petrolatum and 3g of glyceryl behenate in a beaker and stir evenly, heat the above matrix to a viscous fluid state, add 4g of sucrose fatty acid ester (HLB=15) and 5g of hydroxypropyl methylcellulose in turn and stir uniform, add 3 g of ground tinidazole and stir evenly, then heat the above mixture to a viscous fluid state and knead to prepare an ointment.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com