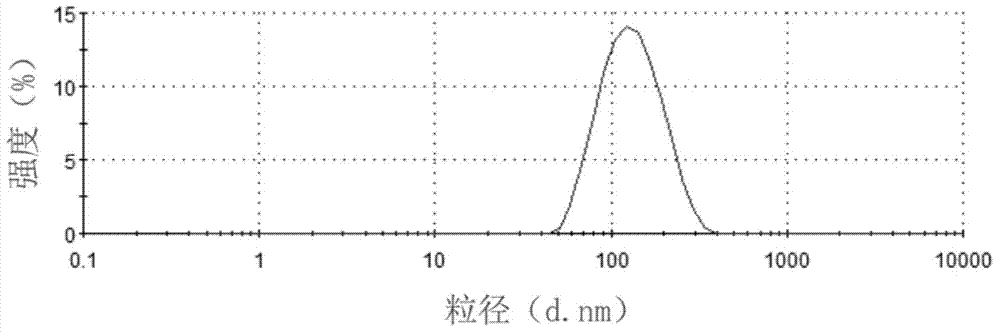
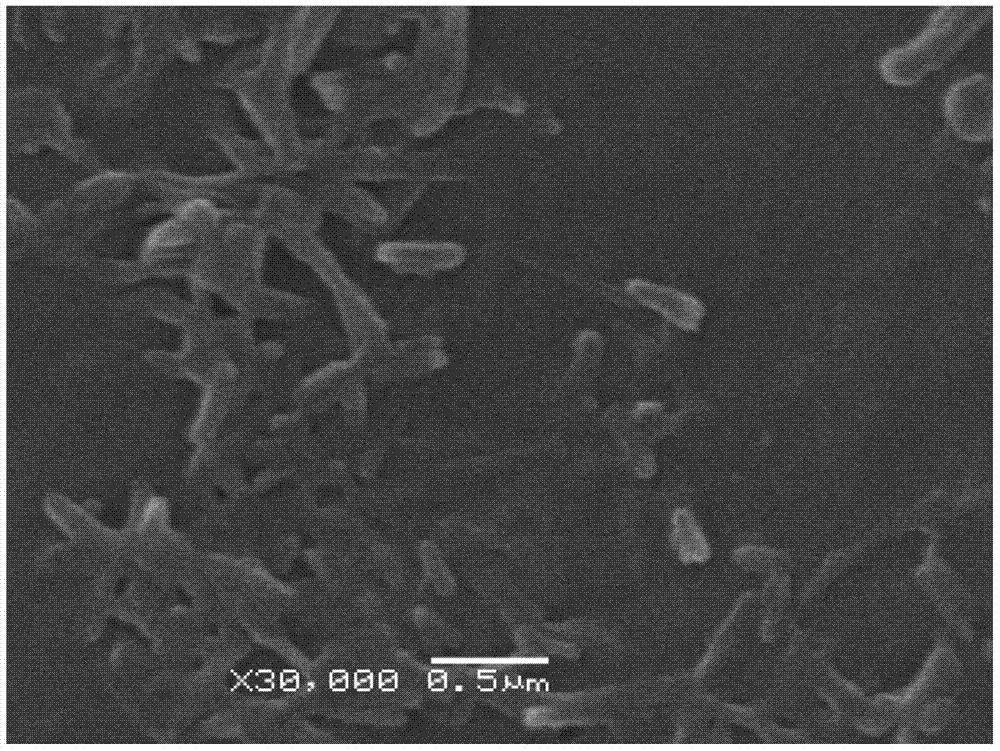
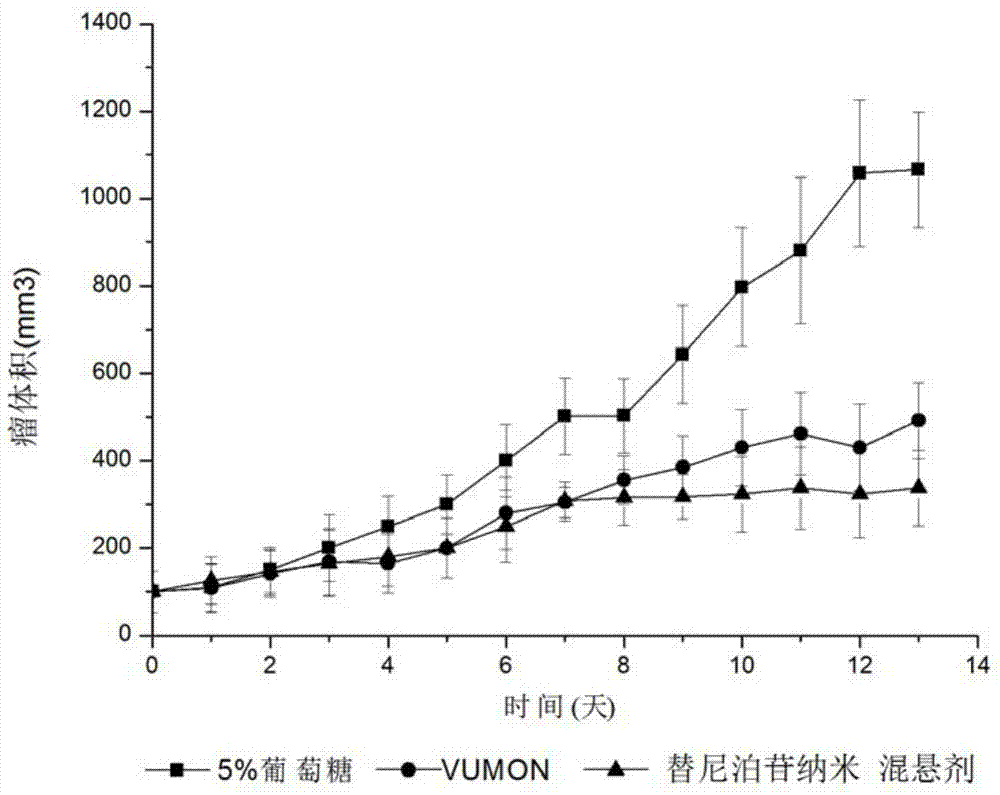
Teniposide nanosuspension and preparation method thereof
A niposide nano- and nano-suspension technology, which is applied in the direction of pharmaceutical formulations, medical preparations containing active ingredients, emulsion delivery, etc., can solve the problems of large toxic and side effects, poor patient compliance, and small drug loading. Achieve the effects of low toxic and side effects, small toxic and side effects, and large drug loading
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] The teniposide nanosuspension of this embodiment contains 5 mg of teniposide and 5 mg of polyvinylpyrrolidone (PVP K30) per 1 ml of the nanosuspension, and the balance is water for injection.
[0049] The preparation method of the teniposide nanosuspension of the present embodiment comprises the following steps:
[0050] 1) Take 500mg of polyvinylpyrrolidone and dissolve it in water for injection to form an aqueous phase; take acetone as organic phase A for subsequent use;
[0051] 2) Dissolve 500 mg of teniposide in organic phase A to form organic phase B;
[0052] 3) Under the condition of stirring at room temperature and rotating speed of 10000rpm, after fully mixing the organic phase B obtained in step 2) with the aqueous phase obtained in step 1), a transparent teniposide emulsion with blue opalescence is formed, and then the acetone is removed by rotary evaporation , and dried in a vacuum drying oven at 40°C for 12 hours to further remove the organic solvent, dil...
Embodiment 2
[0058] The teniposide nanosuspension of this embodiment contains 5 mg of teniposide, 10 mg of polyethylene glycol vitamin E succinate (TPGS) per 1 ml of the nanosuspension, and the balance is water for injection.
[0059] The preparation method of the teniposide nanosuspension of the present embodiment comprises the following steps:
[0060] 1) Dissolve 100 mg of polyethylene glycol vitamin E succinate in water for injection to form an aqueous phase; take methanol as organic phase A for later use;
[0061] 2) Dissolve 50 mg of teniposide in organic phase A to form organic phase B;
[0062] 3) Under the conditions of room temperature and ultrasound (400W), fully mix the organic phase B obtained in step 2) with the aqueous phase obtained in step 1) to form a translucent teniposide emulsion with blue opalescence, and then rotary evaporate Remove methanol, and dry in a vacuum oven at 40°C for 12 hours to further remove the organic solvent, dilute to 10ml with water for injection,...
Embodiment 3
[0067] The teniposide nanosuspension of this embodiment contains 2 mg of teniposide, 1 mg of lecithin, and 0.5 mg of vitamin E per 1 ml of the nanosuspension, and the balance is water for injection.
[0068] The preparation method of the teniposide nanosuspension of the present embodiment comprises the following steps:
[0069] 1) Dissolve 1000mg of lecithin and 500mg of vitamin E in chloroform to form an organic phase A; take water for injection as the water phase and set aside;
[0070] 2) Dissolve 2000mg of teniposide in the organic phase A to form the organic phase B;
[0071] 3) Rotate the organic phase B obtained in step 2) to remove chloroform under reduced pressure, and dry it in a vacuum oven at 35°C for 10 hours to form a thin film; hydrate the thin film with the water phase, vortex it with 400W ultrasound for 4 minutes, and inject it with Dilute to 1000ml with water, and homogenize with a high-pressure homogenizer (20MPa, cycle 5 times) to obtain teniposide nanosus...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com