Method for preparing monosialotetra-hexosylganglioside (GM1) by applying recombinant sialidase converted gangliosides (GLS)
A technology of ganglioside and polysialic acid, which is applied in the field of enzymatic conversion and preparation of monosialotetrahexosyl ganglioside, can solve the problems of complex conversion products, low unit enzyme activity, and human pathogenicity, and achieve The reaction system is simple, the inhibitory effect is released, and the genetic background is clear
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0032] 2.1 Medium
[0033]LB medium: Yeast extract 5g / L; Tryptone 10g / L; NaCl 10g / L, pH 7.0, sterilized at 121°C for 20min.
[0034] Fermentation medium: Yeast extract 5g / L; Tryptone 10g / L; NaCl 10g / L, glycerol 6g / L, 2.4g / L KH 2 PO 4 , 12.5g / L K 2 HPO 4 ·3H 2 O, pH 7.0, sterilized at 121°C for 20min.
[0035] 2.2 Construction of recombinant sialidase plasmid
[0036] 2.2.1 Total synthesis and PCR of Brevibacterium casei sialidase gene
[0037] According to the gene sequence (2925bp, encoding 973 amino acids) of Brevibacterium lactis sialidase published on Genebank with the accession number HQ650539, as shown in SEQ ID NO: 1, the gene sequence of this section was carried out by Shanghai Jierui Bioengineering Co., Ltd. Through total synthesis, the full-length sequence of the Brevibacterium lactis sialidase was obtained.
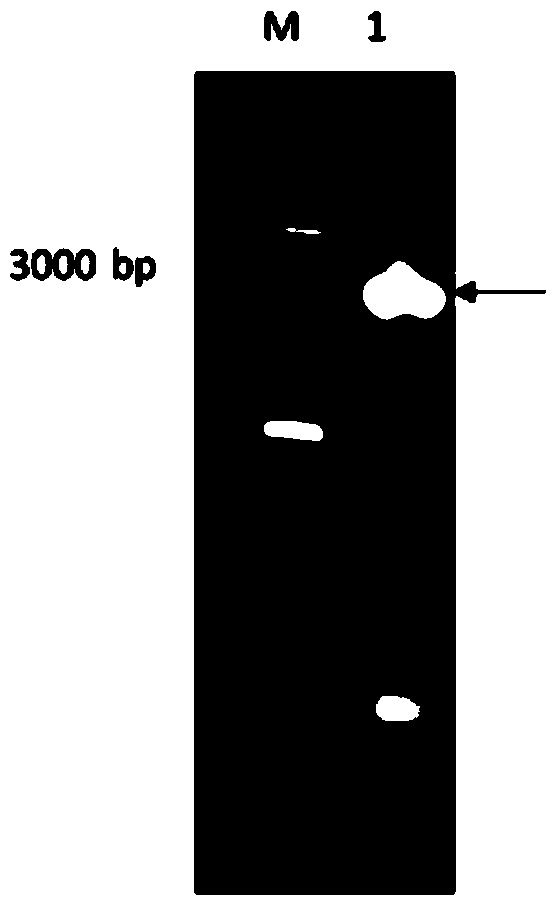
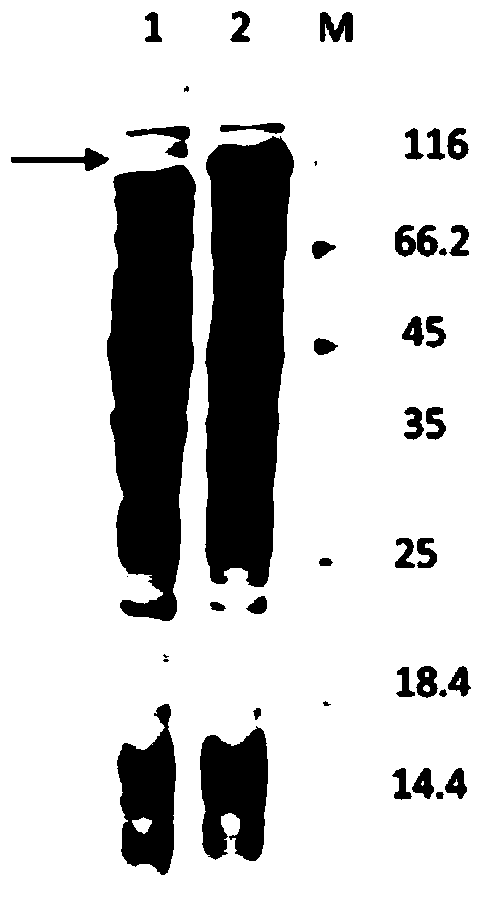
[0038] According to the obtained full-length gene sequence, primers were designed to introduce restriction endonuclease NdeI and HindIII restriction si...
Embodiment 1
[0064] Dialysis bag 10mL reaction system, add 100g / L ganglioside mixture, 10000U / mL recombinant sialidase, 200mL dialysis system, transform for 12h, GLS in ganglioside mixture can be completely converted into GM1. The content of GM1 in the mixture was increased from 8% to 38.17%.
Embodiment 2
[0066] Dialysis bag 100mL reaction system, add 120g / L ganglioside mixture, 10000U / mL recombinant sialidase, 2L dialysis system, transform for 12h, GLS in ganglioside mixture can be completely converted into GM1. The content of GM1 in the mixture was increased from 8% to 36.22%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com