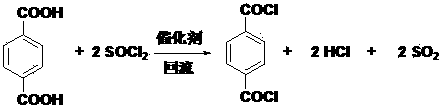
Preparation and purification method of paraphthaloyl chloride with high purity
A terephthaloyl chloride and purification method technology, applied in the field of organic chemistry, can solve the problems of high energy consumption and low material utilization rate, and achieve the effects of improving product purity, convenient operation, and saving energy and material consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] To a three-necked flask equipped with a reflux condenser, a thermometer, and an exhaust gas absorption device, 50 g of terephthalic acid, 90 mL of thionyl chloride, and 3.0 g of 1-hexyl-3-methylimidazolium tetrafluoroborate were sequentially added. The tail gas is absorbed with NaOH alkali solution, start electromagnetic stirring, and heat to 79 o C was refluxed for 10 h, the reactant changed from a white turbid suspension to a light yellow transparent solution, the reaction was terminated, and the product was cooled and precipitated. Filtrate, analyze and detect the content of terephthalic acid, thionyl chloride and catalyst in the filtrate, and add correspondingly according to the amount of reactants to carry out the circular reaction. Add the filtered crude to the melting crystallizer, 65-72 o C is programmed to heat up for 4 hours, release sweat, and raise the temperature to 85 o C, release the product. Obtain 55.2 g of white product, product purity 99.9%, me...
Embodiment 2
[0019] Into a three-necked flask equipped with a reflux condenser, a thermometer, and a tail gas absorption device, 50 g of terephthalic acid, 80 mL of thionyl chloride, 4.5 g of 1-hexyl-3-methylimidazole hexafluorophosphate, and the tail gas Absorb with NaOH alkali solution, start electromagnetic stirring, heat to 89 o C was refluxed for 4 h, the reactant changed from a white turbid suspension to a light yellow transparent solution, the reaction was terminated, and the product was cooled and precipitated. Filtrate, analyze and detect the content of terephthalic acid, thionyl chloride and catalyst in the filtrate, and add correspondingly according to the amount of reactants to carry out the circular reaction. Add filtered crude to melt crystallizer, 72-78 o C program heating for 4 hours, release sweat, and raise the temperature to 90 o C, release the product. Obtain 56.1 g of white product, product purity 99.9%, melting point: 82.0-82.5 o C, yield 91.8%.
[0020] For ...
Embodiment 3
[0022] Into a three-necked flask equipped with a reflux condenser, a thermometer, and an exhaust gas absorption device, add 50 g of terephthalic acid, 80 mL of thionyl chloride, and 3.5 g of 1-butyl-3-methylimidazolium tetrafluoroborate in sequence. , the tail gas is absorbed with NaOH alkali solution, start electromagnetic stirring, and heat to 80 o C was refluxed for 10 h, the reactant changed from a white turbid suspension to a light yellow transparent solution, the reaction was terminated, and the product was cooled and precipitated. Filtrate, analyze and detect the content of terephthalic acid, thionyl chloride and catalyst in the filtrate, and add correspondingly according to the amount of reactants to carry out the circular reaction. The filtered crude product was distilled under reduced pressure, the vacuum degree was 133 Pa, and the fraction at 105-110°C was received to obtain 58.0 g of a white product with a purity of 99.9% and a melting point of 82.5-83.0 o C, yiel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com