Method for covering lithium iron phosphate conducting layer by use of radio frequency plasma enhanced chemical vapor deposition
A technology of radio frequency plasma and lithium iron phosphate, applied in electrochemical generators, circuits, electrical components, etc., can solve the problems of long time, high energy consumption, high heat treatment temperature of the coated metal conductive layer, etc., and achieve short reaction time, The effect of low reaction temperature and good crystal structure development
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
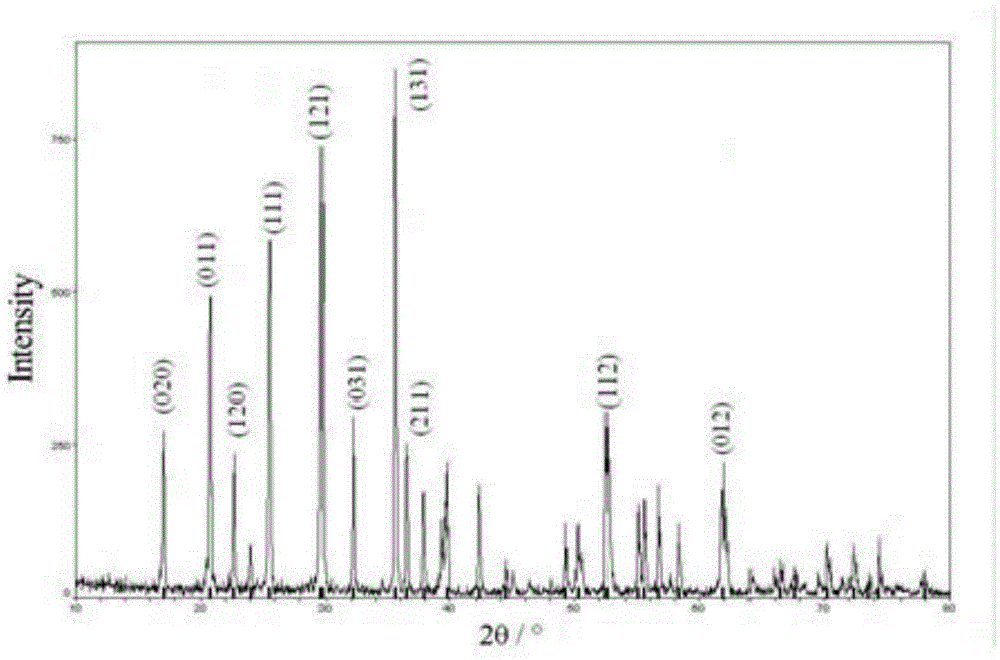
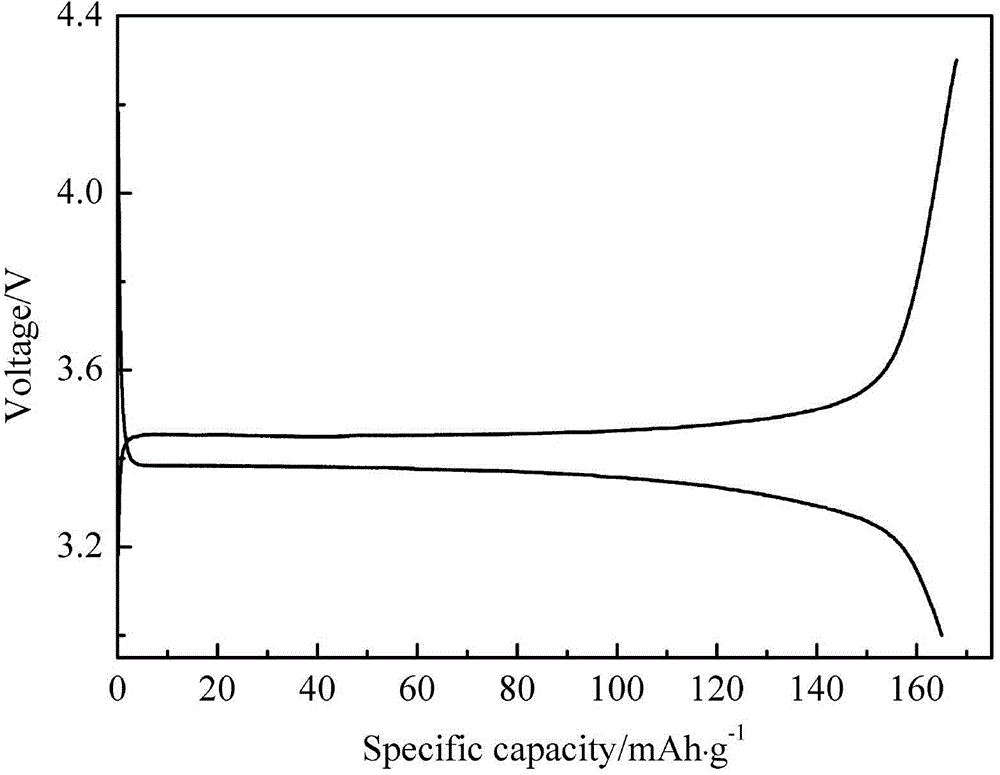
[0020] Weigh ferrous phosphate, lithium hydroxide, and phosphoric acid according to the molar ratio Li:Fe:P=1:1:1, dissolve them in an appropriate amount of deionized water, and transfer them to a polytetrafluoroethylene hydrothermal reaction tank, and seal them. Put the reaction tank in a blast drying oven, and conduct a hydrothermal reaction at 160°C for 48 hours under the blast state. After natural cooling, use deionized water to wash repeatedly by centrifugation, and vacuum-dry the separated powder at 80°C for 10 hours. Obtain lithium iron phosphate. The prepared sample LiFePO 4 Placed in Shenyang Xinlantian Plasma Enhanced Chemical Vapor Deposition System. Vacuum down to 1×10 -1 Pa. Filled with SiH 4 +H 2 (SiH 4 :H 2 =1:9 volume ratio) gas, the flow rate is controlled at 40sccm, and the total pressure of the reaction chamber is maintained at 10Pa. The output power of the RF plasma power supply is 200W. The reaction time was controlled at 30min. After the reactio...
Embodiment 2
[0022] Weigh ferrous phosphate, lithium acetate, and phosphoric acid according to the molar ratio Li:Fe:P=1:1:1, dissolve them with appropriate amount of deionized water, and transfer them to a polytetrafluoroethylene hydrothermal reaction tank, and seal them. Place the reaction tank in a blast drying oven, and conduct a hydrothermal reaction at 180°C for 24 hours under the blast state. After natural cooling, use deionized water to repeatedly centrifuge and clean, and vacuum-dry the separated powder at 80°C for 5 hours. Obtain lithium iron phosphate. The prepared sample LiFePO 4 Placed in Shenyang Xinlantian Plasma Enhanced Chemical Vapor Deposition System. Vacuum down to 1×10 -1 Pa. charge GeH 4 +Ar(GeH 4 :Ar=1:20 volume ratio) gas, the flow rate is controlled at 100sccm, and the total pressure of the reaction chamber is maintained at 200Pa. The output power of the RF plasma power supply is 100W. The reaction time was controlled at 60min. After the reaction is complet...
Embodiment 3
[0024] Weigh ferrous sulfate, lithium chloride, and ammonium dihydrogen phosphate according to the molar ratio Li:Fe:P=1:1:1, dissolve them in appropriate amount of deionized water, and transfer them to a polytetrafluoroethylene hydrothermal reaction tank, seal . Place the reaction tank in a blast drying oven, and conduct a hydrothermal reaction at 200°C for 18 hours under the blast state. After natural cooling, use deionized water to wash repeatedly by centrifugation, and vacuum-dry the separated powder at 80°C for 15 hours. Obtain lithium iron phosphate. The prepared sample LiFePO 4 Placed in Shenyang Xinlantian Plasma Enhanced Chemical Vapor Deposition System. Vacuum down to 1×10 -1 Pa. Charge BH 3 +N 2 (BH 3 :N 2 =1:15 volume ratio) gas, the flow rate is controlled at 70sccm, and the total pressure of the reaction chamber is maintained at 500Pa. The output power of the RF plasma power supply is 60W. The reaction time was controlled at 90min. After the reaction i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com