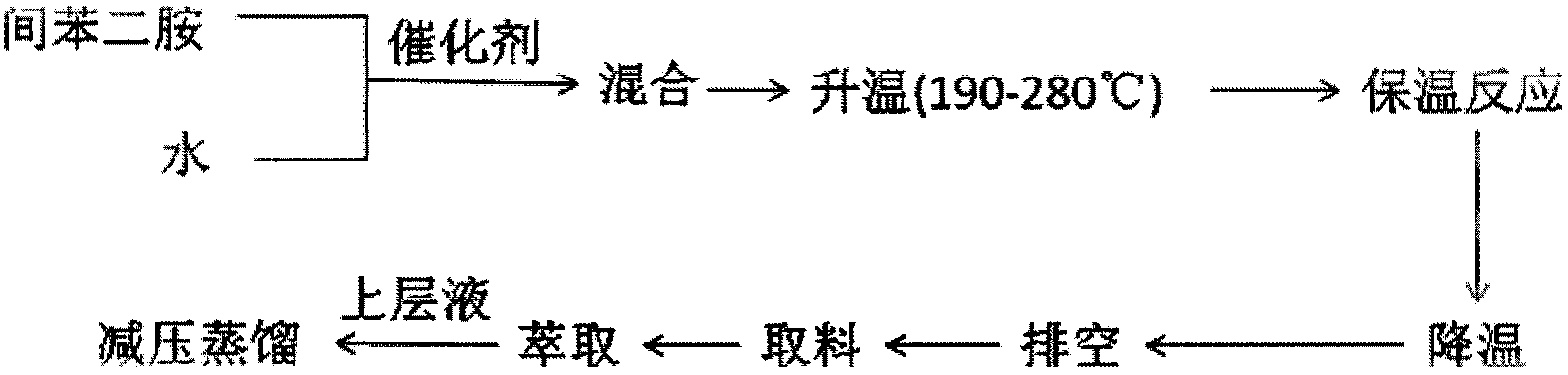
Process of producing resorcinol by hydrolyzing m-phenylenediamine
A technology of m-phenylenediamine and resorcinol, which is applied in the field of resorcinol preparation, can solve the problems of oxidation, complex extraction process, lengthy production process, and low environmental friendliness, so as to reduce the generation of coke, The effect of high product yield and low environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] In a 5L autoclave equipped with stirring (heated by an electric heating coil), add 216.0g m-phenylenediamine, 350.0g hydrochloric acid (concentration 30%), 2200g water, 27.94g SO 4 2- / ZrO 2 -TiO 2 , The temperature of the feed liquid does not exceed 80°C. Stir for 30 minutes after the addition to make the materials evenly mixed. After airtight, heat to 200°C, pressure 1.8MPa, heat preservation reaction for 10 hours, after the reaction, cool down to room temperature 30°C; take out the reaction solution, filter, extract 4 times with 250.0g ether, distill off the solvent to obtain 175.0g of crude product, content It is 95.50%, obtains product 162.0g through distillation under 1.8KPa, and content is 99.38%.
Embodiment 2
[0025] In a 5L autoclave equipped with stirring (heated by an electric heating coil), add 220.8g m-phenylenediamine, 368.0g sulfuric acid (concentration 98%), 2600g water, 354.31g SO 4 2- / ZrO 2 -TiO 2 , The temperature of the feed liquid does not exceed 80°C. Stir for 30 minutes after the addition to make the materials evenly mixed. After airtight, heat to 220°C, pressure is 2.2MPa, heat preservation reaction for 8 hours, after the reaction, cool down to room temperature 30°C; take out the reaction solution, filter, extract 4 times with 400g ethyl acetate, distill off the solvent to obtain 185.0g of crude product, The content is 95.30%, and the product 178.0g is obtained through distillation at 2.0KPa, and the content is 99.40%.
Embodiment 3
[0027] In a 5L autoclave equipped with stirring (heated by an electric heating coil), add 426.0g m-phenylenediamine, 240.0g hydrochloric acid (concentration 30%), 2600g water, 40.0g SO 4 2- / TiO 2 -SnO 2 , The temperature of the feed liquid does not exceed 80°C. Stir for 30 minutes after the addition to make the materials evenly mixed. After airtight, heat to 200°C, pressure is 2MPa, heat preservation reaction for 4 hours, after the reaction, cool down to room temperature 30°C; take out the reaction solution, filter, extract 4 times with 500g isobutanol, distill off the solvent to obtain 295.0g of crude product, content It is 95.25%, obtains 281.0g of product through distillation at 1.9KPa, and content is 99.50%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com