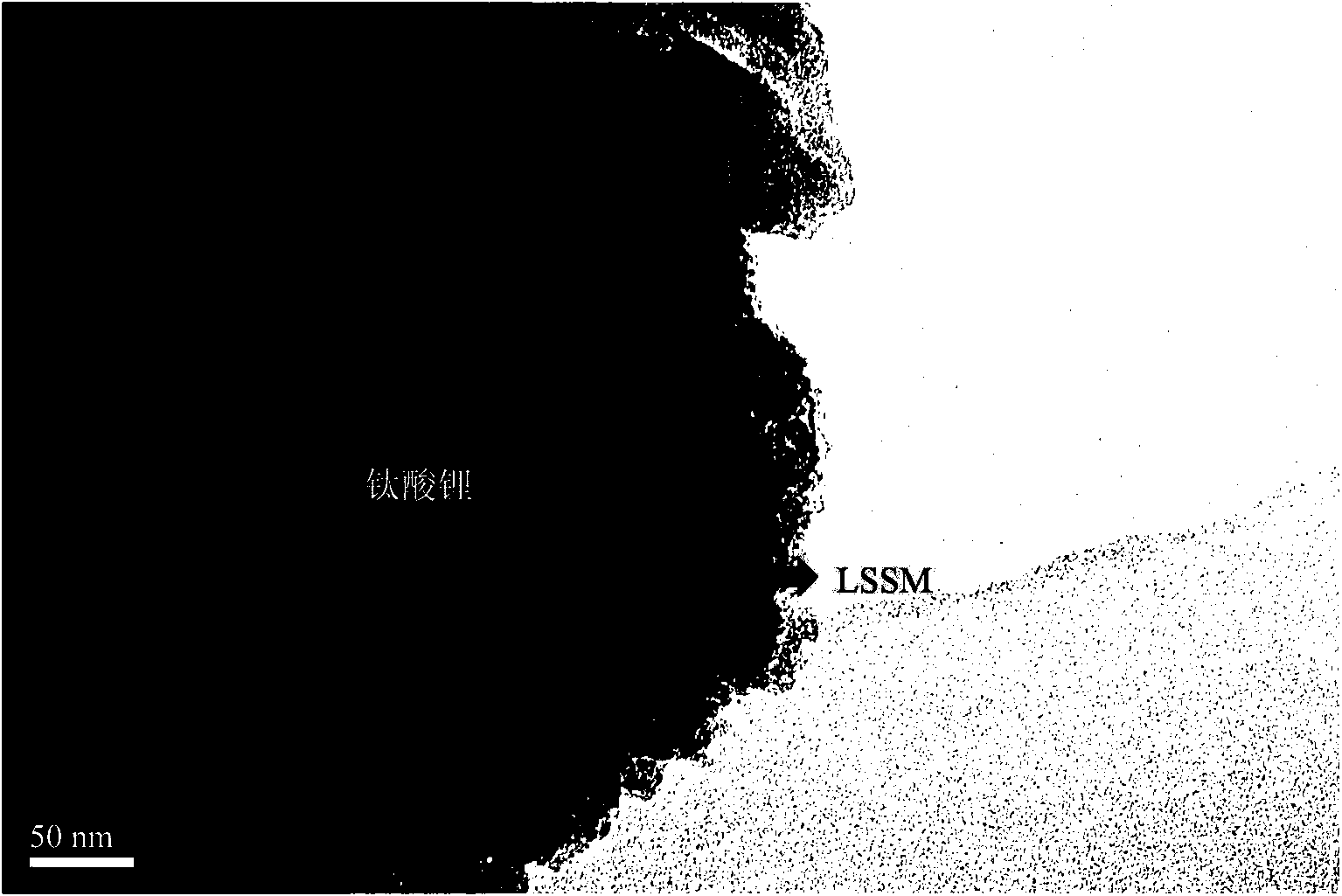
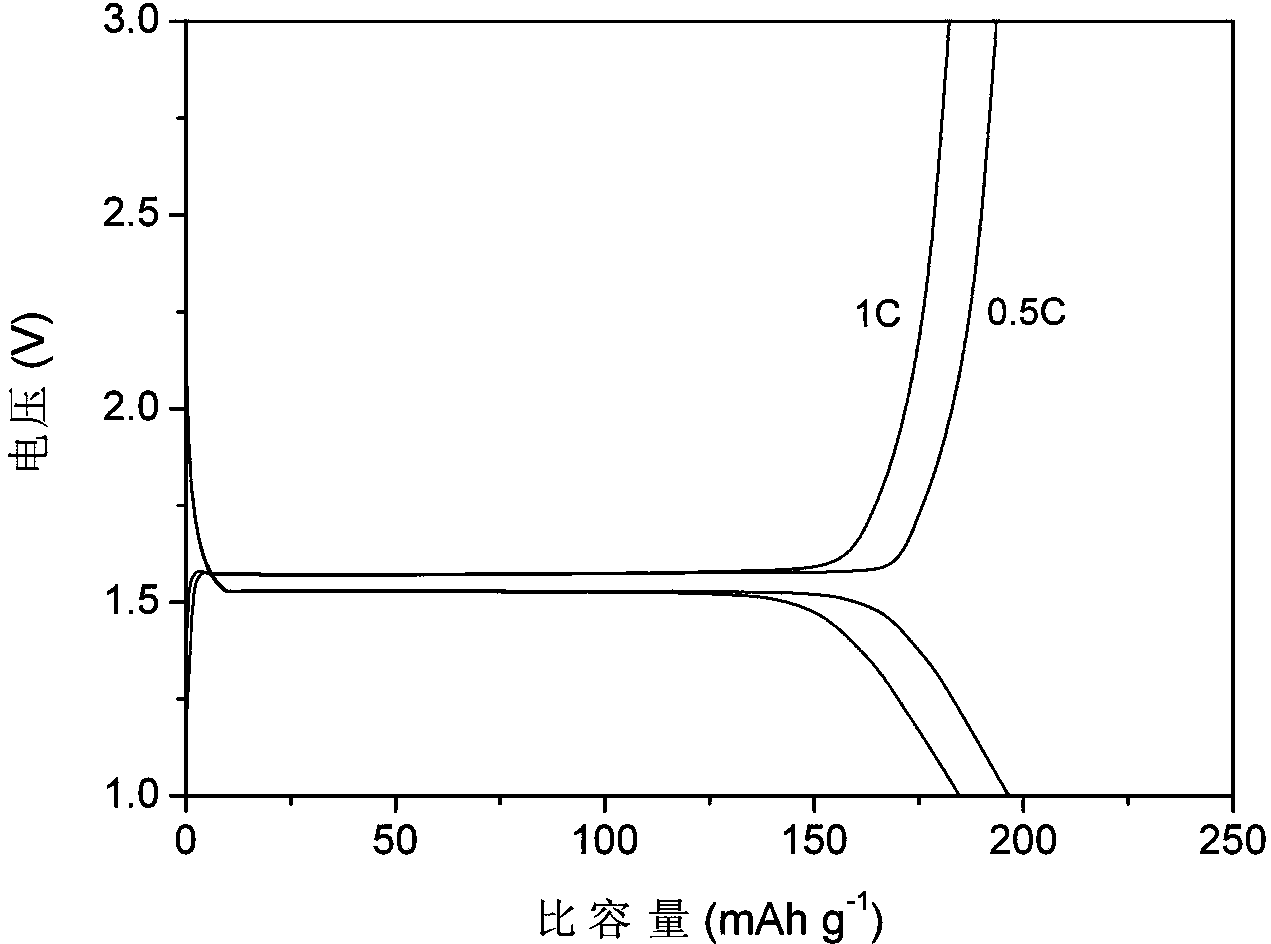
Preparation method of nanometer lithium titanate covered with double highly-conductive materials
A high-conductivity, lithium titanate technology, applied in nanotechnology, circuits, electrical components, etc., can solve the problems of electrolyte consumption, low first-time Coulombic efficiency, low first-time charge-discharge efficiency, limitations, etc., and achieve superior fast charge-discharge performance , Improve the effect of particle agglomeration and good cycle stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] First, premix 300 ml of ethanol and 30 ml of water according to the volume ratio of 1:0.1 to form a mixed solvent, and then add 15 ml of HNO 3As an inhibitor of the follow-up reaction; the soluble compounds of Li and Ti are compounded according to the molar ratio of Li:Ti=4.2:5, and 25.52g of tetra-n-butyl titanate (analytically pure) is weighed, and 2.33g of carbonic acid Lithium (analytical pure), added to the previous alcohol-water-acid mixture, stirred by a magnetic heating stirrer until completely dissolved; then 20g of ethylenediaminetetraacetic acid and 30g of citric acid were added to the pre-mixed metal ion solution After mixing evenly, add 100 ml of ammonia water dropwise to adjust the pH value to 8, and continue to stir; after the above mixed solution is stirred evenly to form a sol, heat and stir at 80°C until it reaches a gel state; :Sr:Sc:Mn=0.5:0.5:0.1:0.9 molar ratio for batching, weigh 9.48 g lanthanum acetate (analytical pure), 9.69 g strontium nitrate...
Embodiment 2
[0030] According to the volume ratio of 1:0.1, 300 ml of ethanol and 30 ml of water are premixed to form a mixed solvent, and then 15 ml of HCl is added as an inhibitor of the subsequent reaction; the soluble compound of Li and Ti is compounded according to Li:Ti=4.2:5 The molar ratio is carried out batching, and the tetraisopropyl titanate (analytical pure) of 21.31g, the lithium acetate (analytical pure) of 6.43 g are joined in the previous alcohol-water-acid mixture, stirred by a magnetic heating stirrer, Until it is completely dissolved; then add 20 g of ethylenediaminetetraacetic acid and 40 g of citric acid into the premixed metal ion solution, mix well, add 100 ml of ammonia water dropwise to adjust the pH value to 9, and continue stirring; wait for the above mixing After the solution was stirred evenly to form a sol, it was then heated and stirred at 80°C until it reached a gel state; the La, Sr, Sc and Mn compounds were mixed according to the molar ratio of La:Sr:Sc:Mn...
Embodiment 3
[0032] According to the volume ratio of 1:0.2, pre-mix 300 ml of ethanol and 60 ml of water to form a mixed solvent, and then add 30 ml of HNO 3 As an inhibitor of the follow-up reaction; the soluble compounds of Li and Ti are compounded according to the molar ratio of Li:Ti=4.4:5, and 25.52 g of tetra-n-butyl titanate (analytical pure), 4.55 g of nitric acid Lithium (analytically pure), was added to the previous alcohol-water-acid mixture, stirred by a magnetic heating stirrer until it was completely dissolved; then 20 g of ethylenediaminetetraacetic acid and 60 g of citric acid were added to the pre-mixed metal In the ionic solution, after mixing evenly, add 110ml of ammonia water dropwise to adjust the pH value to 7, and continue to stir; after the above mixed solution is stirred evenly to form a sol, heat and stir at 80°C until it is in a gel state; La:Sr:Sc:Mn=0.5:0.5:0.1:0.9 molar ratio for batching, weigh 9.48 g lanthanum oxalate (analytical pure), 5.27 g strontium oxal...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com