Novel DPP-4 inhibitor, and preparation method and medicinal application thereof
A pharmacy and mixed solvent technology, which is applied in the field of preparation of anti-diabetes and its complication drugs and DPP-4 inhibitors, can solve the problems that the application prospect of alogliptin remains to be seen, and achieves short preparation route, cheap and easy-to-obtain raw materials, The effect of simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
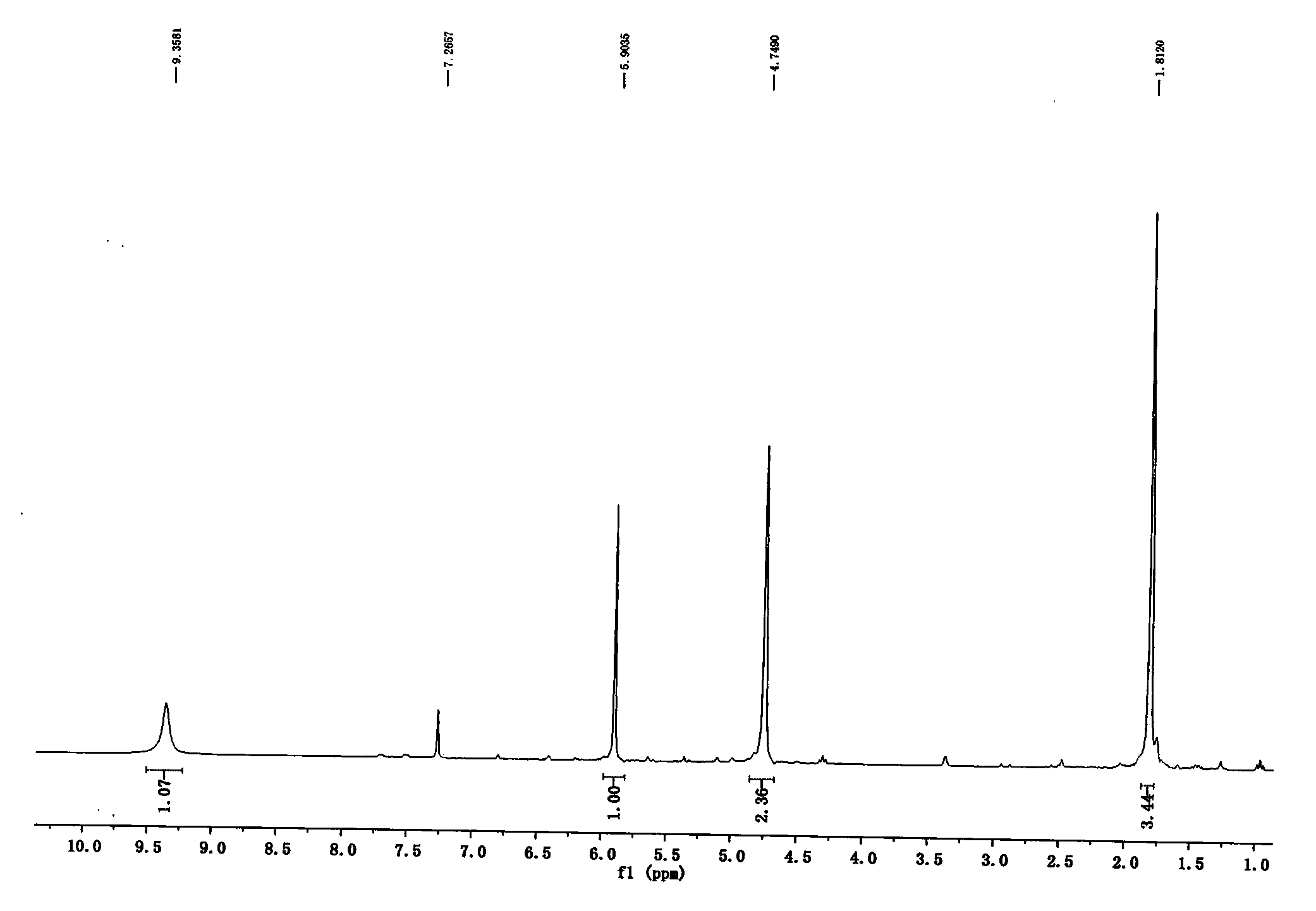
[0029] Example 1: Synthesis of 1-(2-butyn-1-yl)-6-chloro-2,4-(1H,3H)pyrimidinedione (III)
[0030] To 6-chlorouracil (71g, 480mmol), add 200ml DMF, DIPEA (110ml, 624mmol), cool to 0°C, dropwise add 1-bromo-2-butyne (48ml, 528mmol), stir overnight at room temperature, add water 200ml of precipitated solid was filtered, washed with water, washed with ethyl acetate, and dried to obtain 81g of off-white solid with a yield of 85%. mp: 216-217°C; 1 H NMR (300MHz, CDCl 3 )δ9.36(s, 1H), 5.90(s, 1H), 4.75(s, 2H), 1.81(s, 3H).HRMS-ESI(m / z) calcd for C 8 h 8 N 2 o 2 Cl[M+H] + : 199.0274, found 199.0276
Embodiment 2
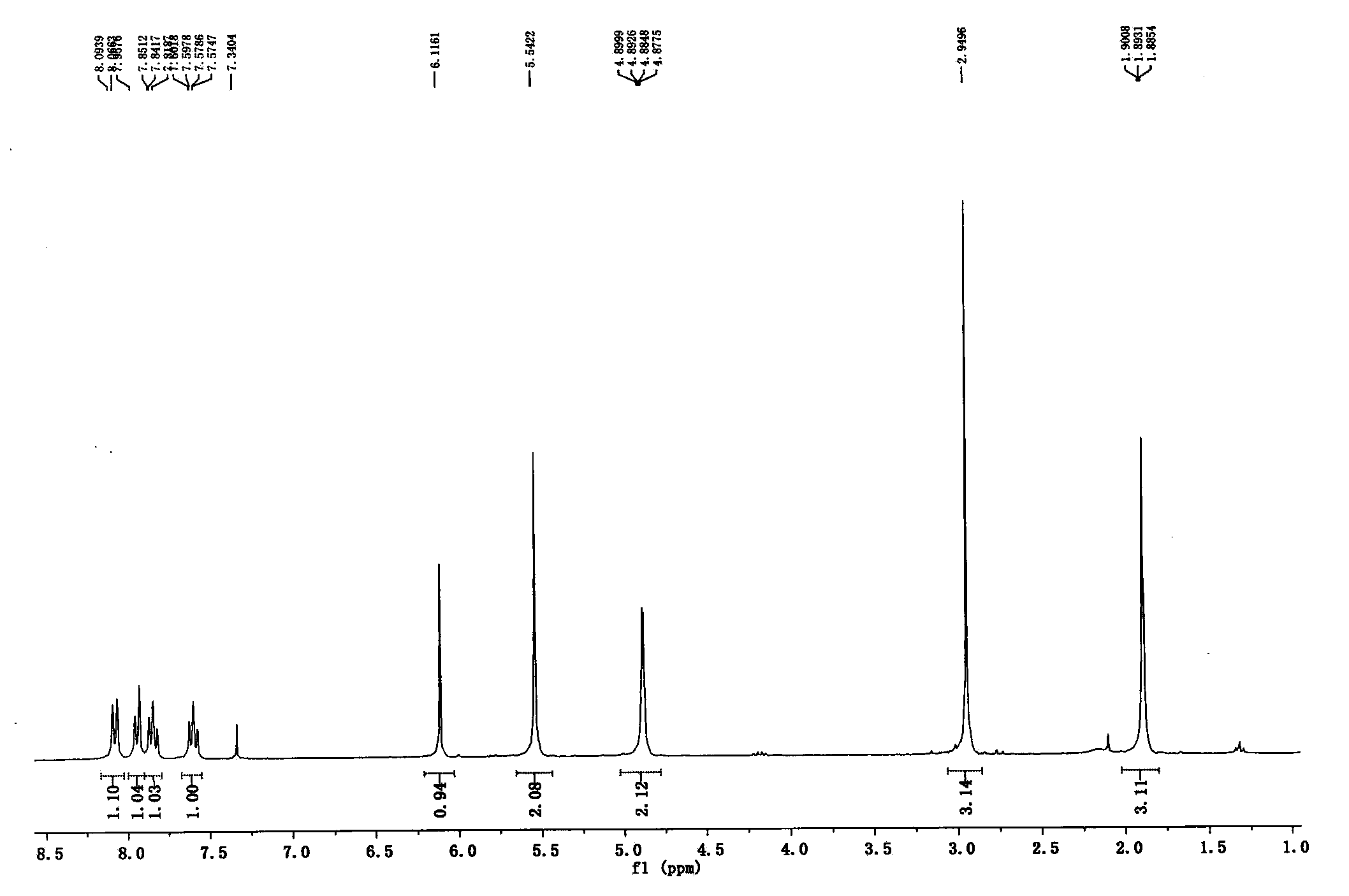
[0031] Example 2: 1-(2-butyn-1-yl)-6-chloro-3-[(4-methylquinazolin-2-yl)methyl]-2,4-(1H,3H) Pyrimidinedione (IV)
[0032]Take 1-(2-butyn-1-yl)-6-chloro-2,4-(1H,3H)pyrimidinedione (III) (14.6g, 73.5mmol), 2-chloromethyl-4-form Quinazoline (15.6g, 80.8mmol), lithium bromide (5.69g, 58.8mmol), 60% sodium hydride (3.82g, 95.6mmol), after adding DMF 150ml, react overnight at 80°C under a nitrogen atmosphere, and remove by distillation under reduced pressure Add 100ml of ethyl acetate and 200ml of isopropyl ether to DMF, cool to -20°C to precipitate a solid, filter, wash with ethyl acetate and isopropyl ether, and obtain 21g of a light yellow solid with a yield of 80%. mp: 153°C; 1 H NMR (300MHz, CDCl 3 )δ8.08 (d, J=8.3Hz, 1H), 7.94 (d, J=8.2Hz, 1H), 7.85 (ddd, J=8.4, 6.9, 1.3Hz, 1H), 7.60 (ddd, J=8.1 , 6.9, 1.2Hz, 1H), 6.12(s, 1H), 5.54(s, 2H), 4.89(dd, J=4.5, 2.2Hz, 2H), 2.95(s, 3H), 1.89(t, J= 2.3Hz,3H).HRMS-ESI(m / z)calcd for C 18 h 16 N 4 o 2 Cl[M+H] + : 355.0962, foun...
Embodiment 3
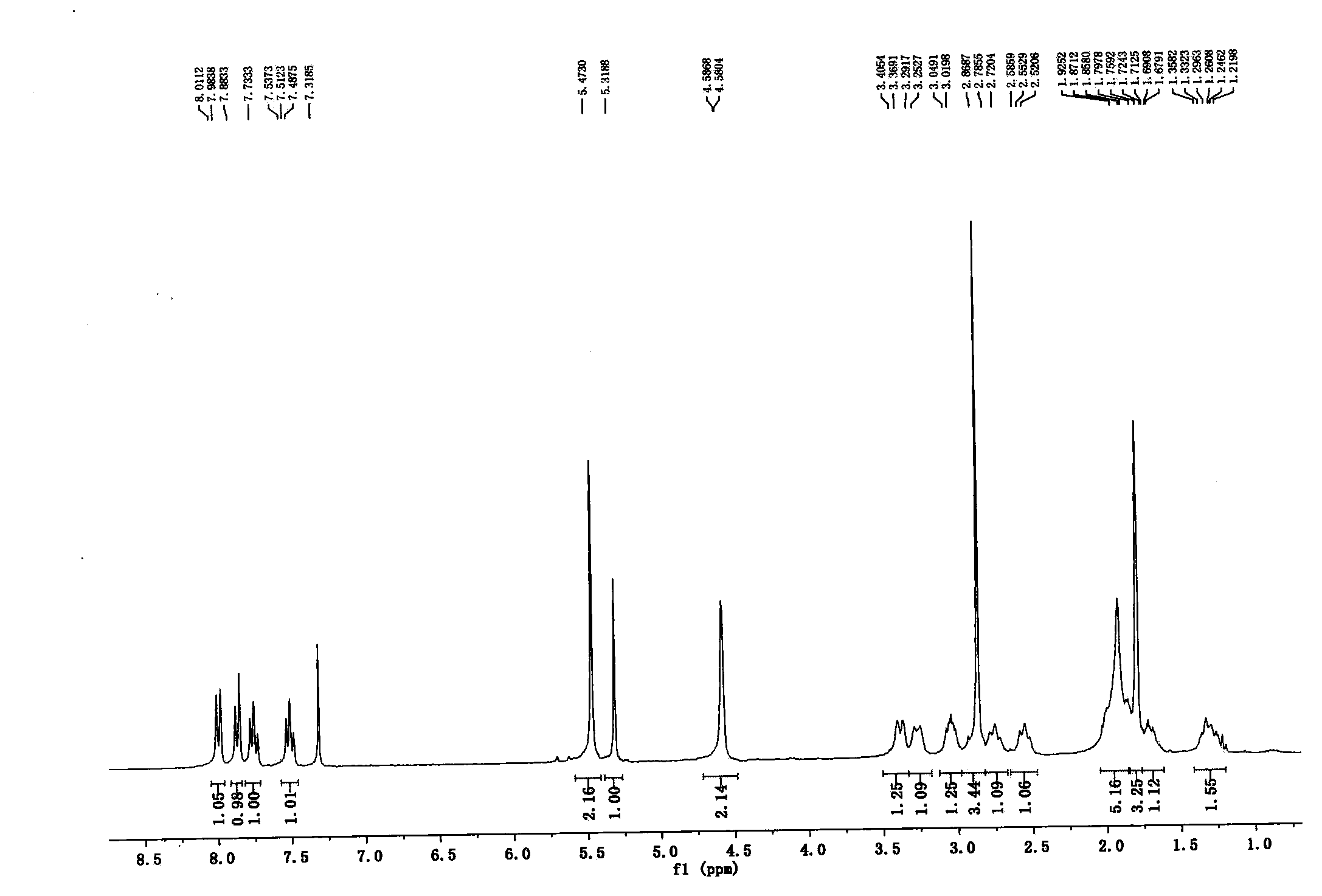
[0033] Example 3: 1-(2-butyn-1-yl)-6-[3(R)-aminopiperidin-1-yl]-3-[(4-methylquinazolin-2-yl) Methyl]-2,4-(1H,3H)pyrimidinedione (I)
[0034] Take 1-(2-butyn-1-yl)-6-chloro-3-[(4-methylquinazolin-2-yl)methyl]-2,4-(1H,3H)pyrimidinedione (IV) (8 g, 22.5 mmol), NaHCO 3 (9.45g, 112.5mmol), 4A molecular sieves (2.6g), (R)-3-aminopiperidine dihydrochloride (4.67g, 27mmol), add 150ml isopropanol, react for 2 hours at 100°C, cool to At room temperature, the solid was removed by filtration, concentrated under reduced pressure, and column chromatography (dichloromethane / methanol / triethylamine 100:0.5:0.5) yielded 8 g of light yellow solid, yield 85%. 1 H NMR (300MHz, CDCl 3 )δ8.00(d, J=8.2Hz, 1H), 7.87(d, J=8.3Hz, 1H), 7.76(t, J=7.5Hz, 1H), 7.51(t, J=7.5Hz, 1H) , 5.47(s, 2H), 5.32(s, 1H), 4.58(d, J=1.9Hz, 2H), 3.39(d, J=10.9Hz, 1H), 3.27(d, J=11.7Hz, 1H) , 3.13-2.98(m, 1H), 2.98-2.82(m, 3H), 2.76(dd, J=15.4, 6.2Hz, 1H), 2.65-2.47(m, 1H), 2.05-1.86(m, 5H) , 1.80(s, 3H), 1.76-1.62(m,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com