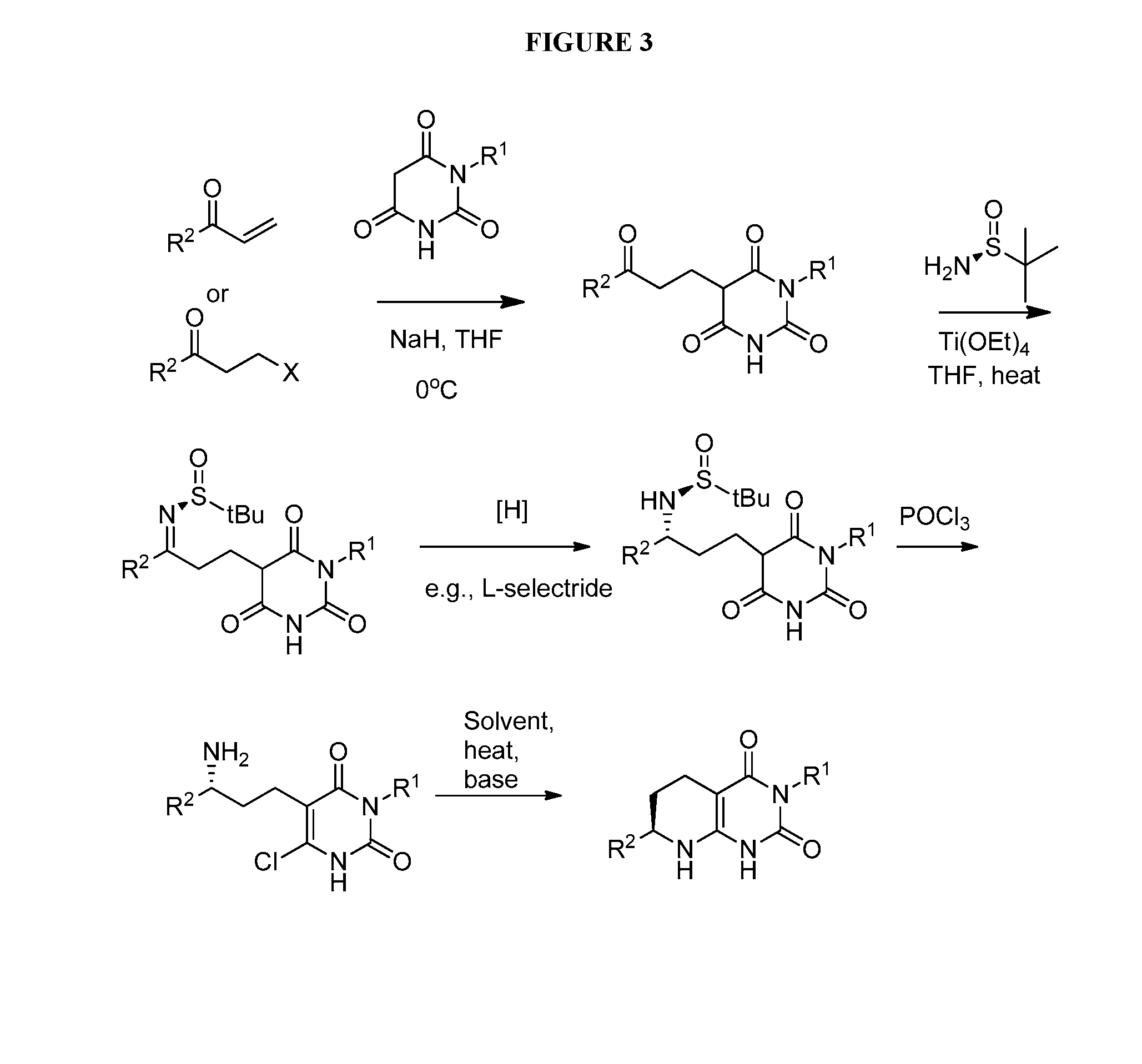
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
123 results about "Pyrimidinedione" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Pyrimidinediones are a class of chemical compounds characterized by a pyrimidine ring substituted with two carbonyl groups.
N-Substituted Glycine Derivatives: Prolyl Hydroxylase Inhibitors
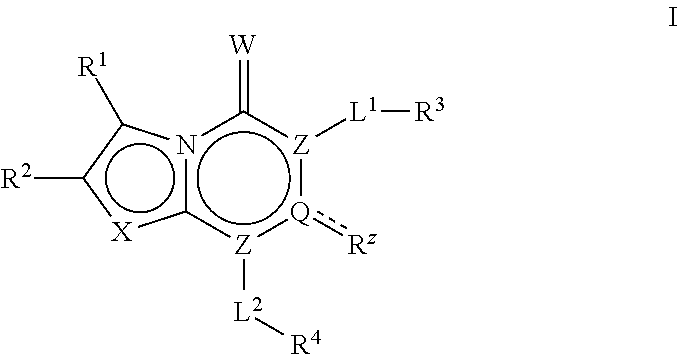
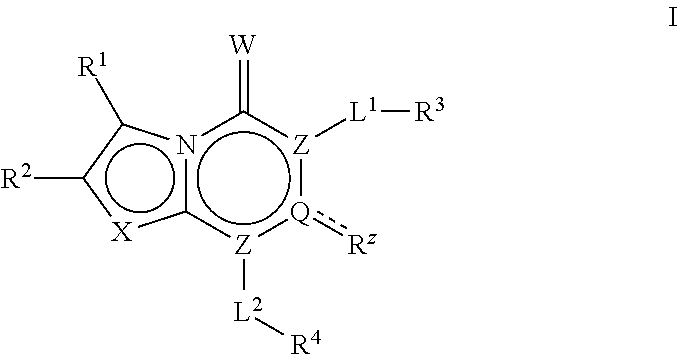
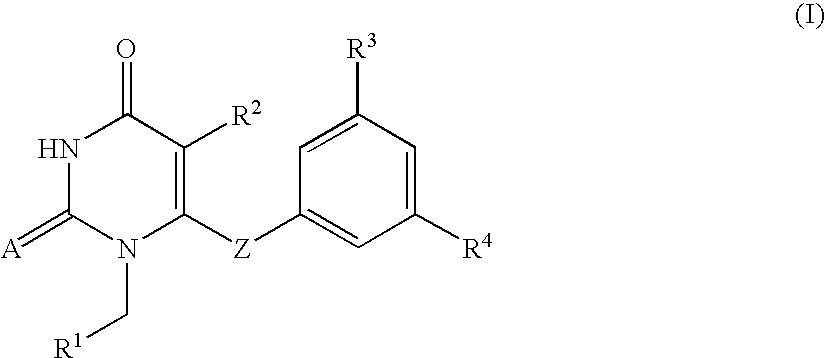
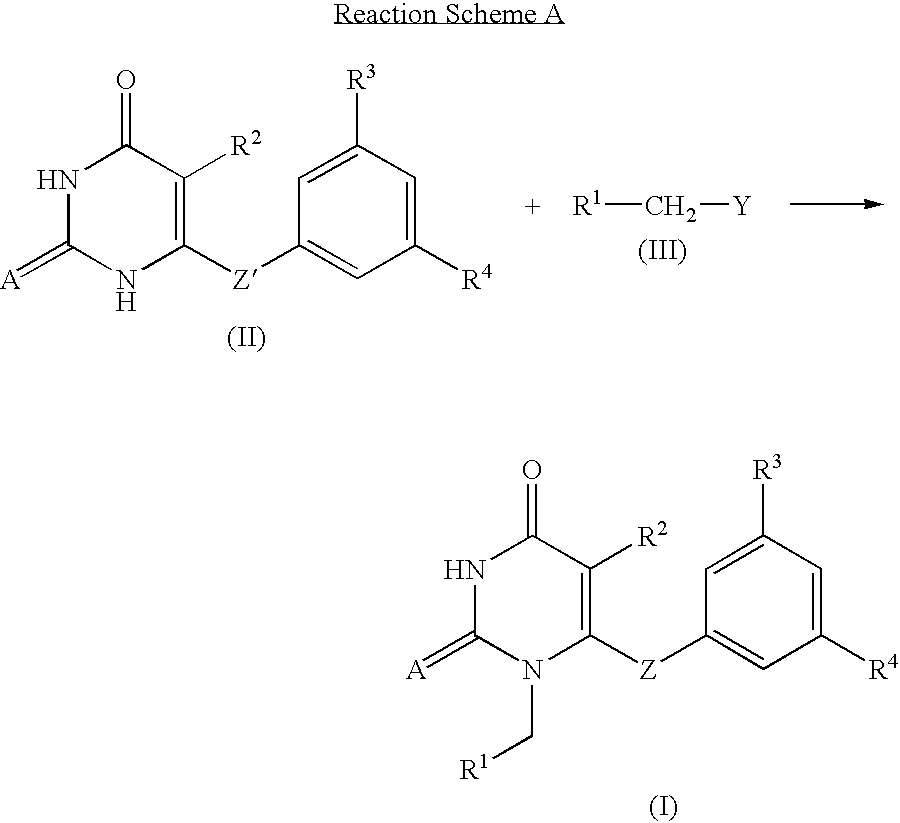
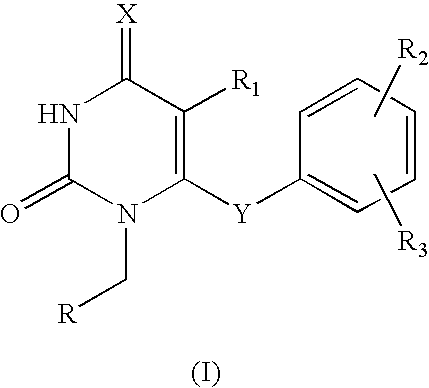
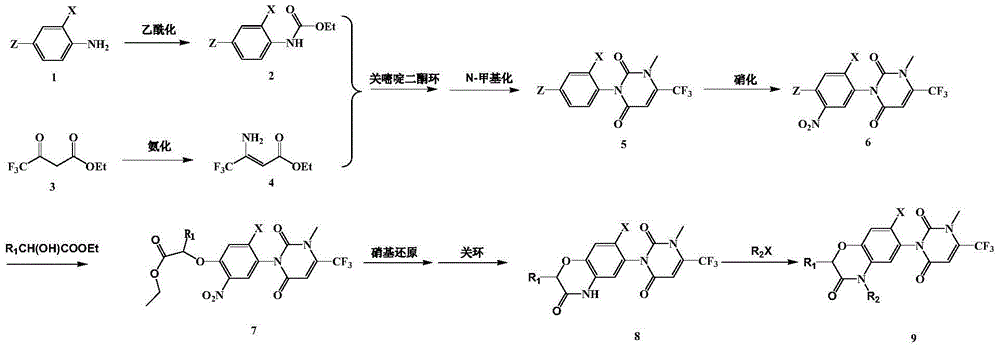
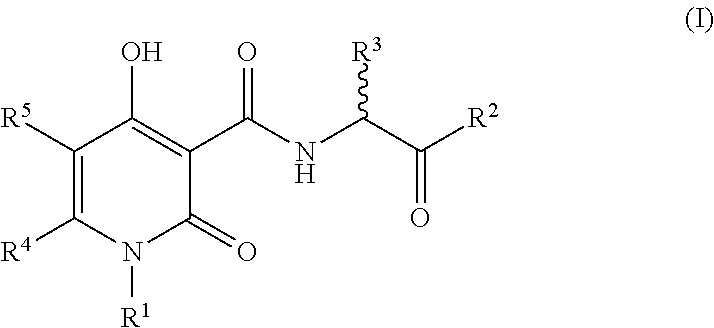
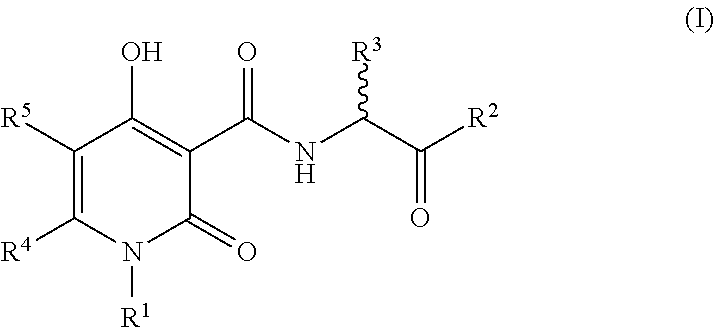
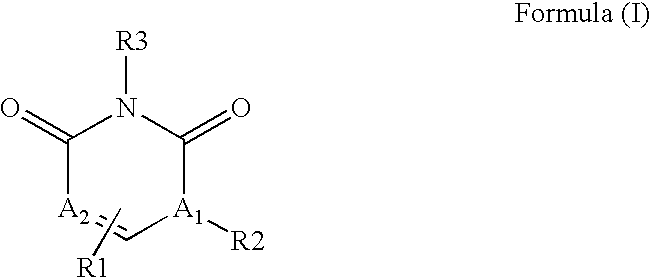
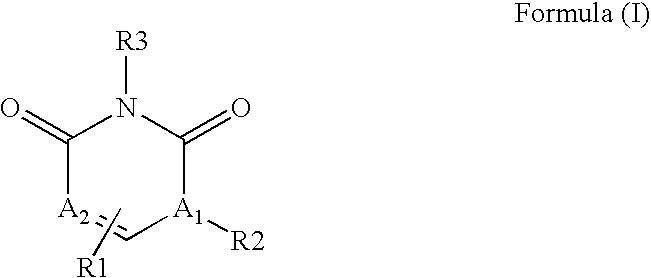
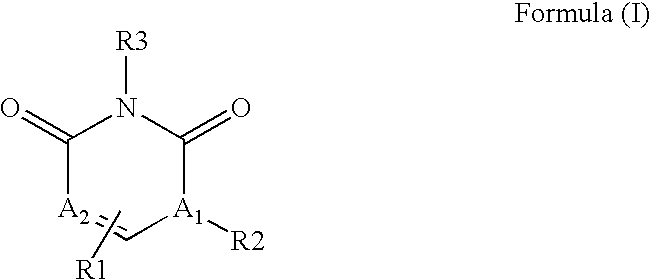
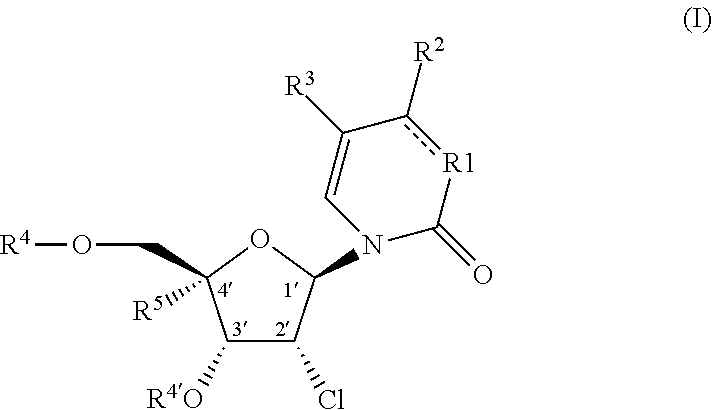
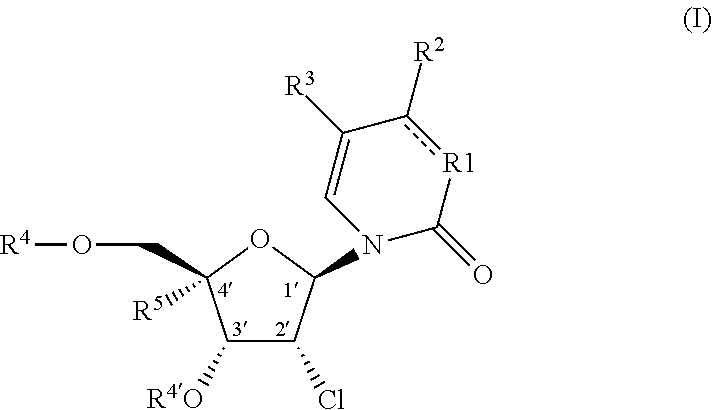
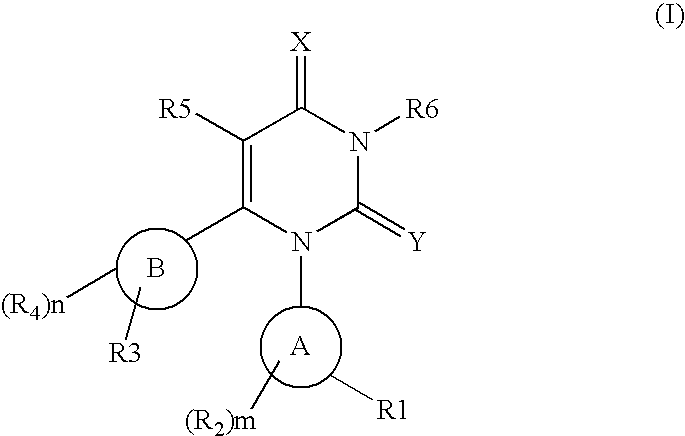
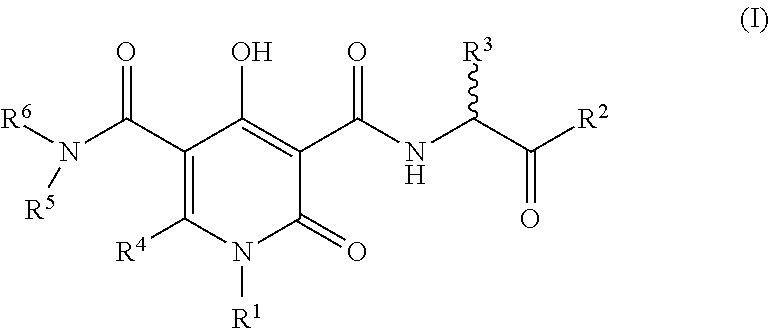
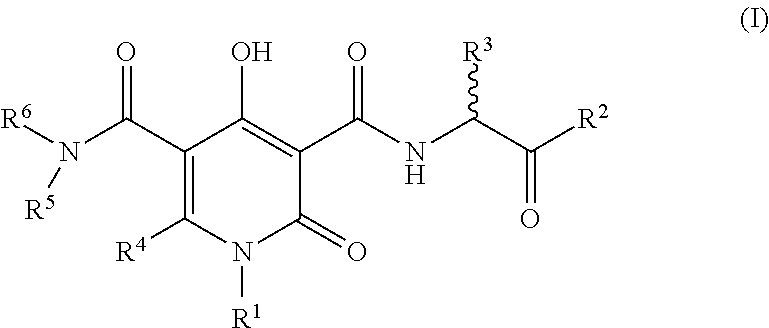
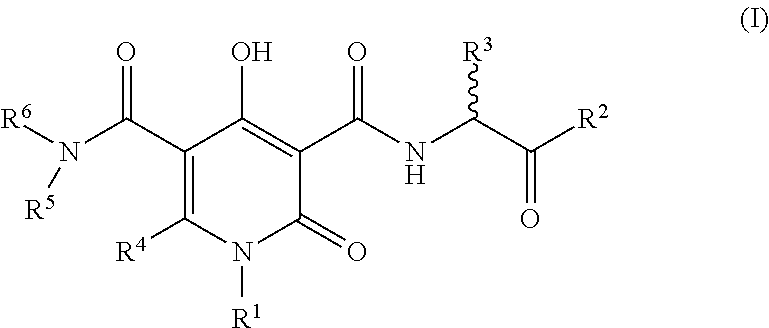
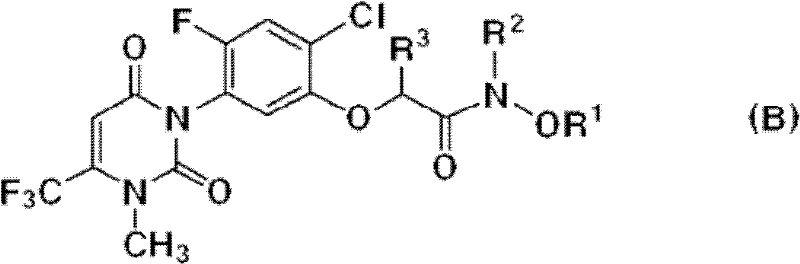
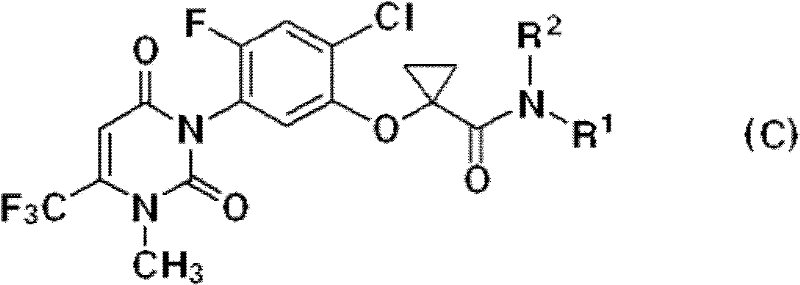
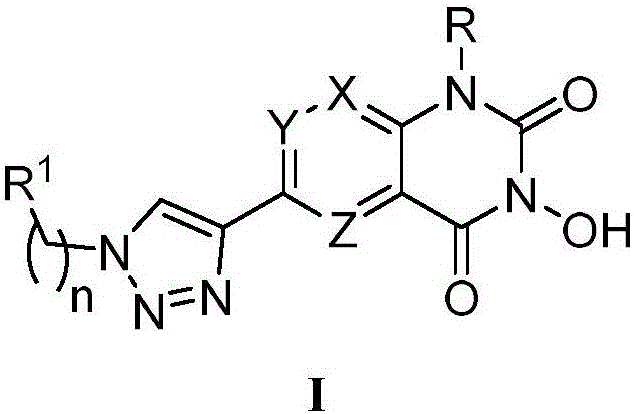
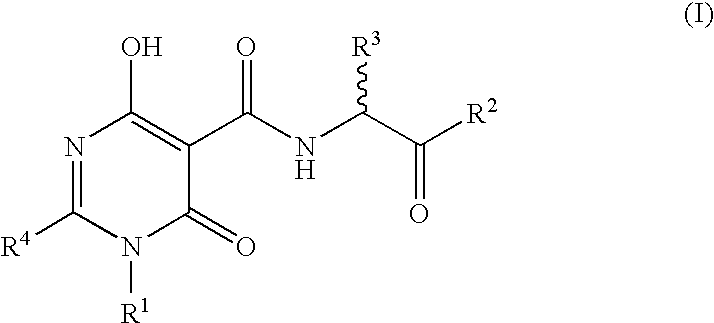
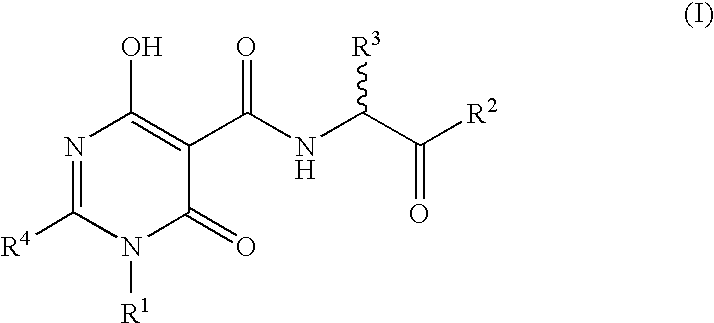
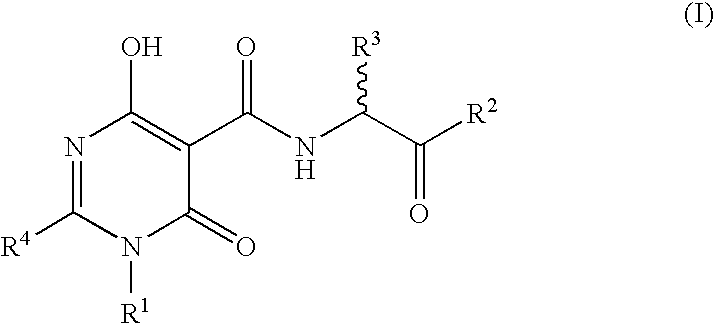
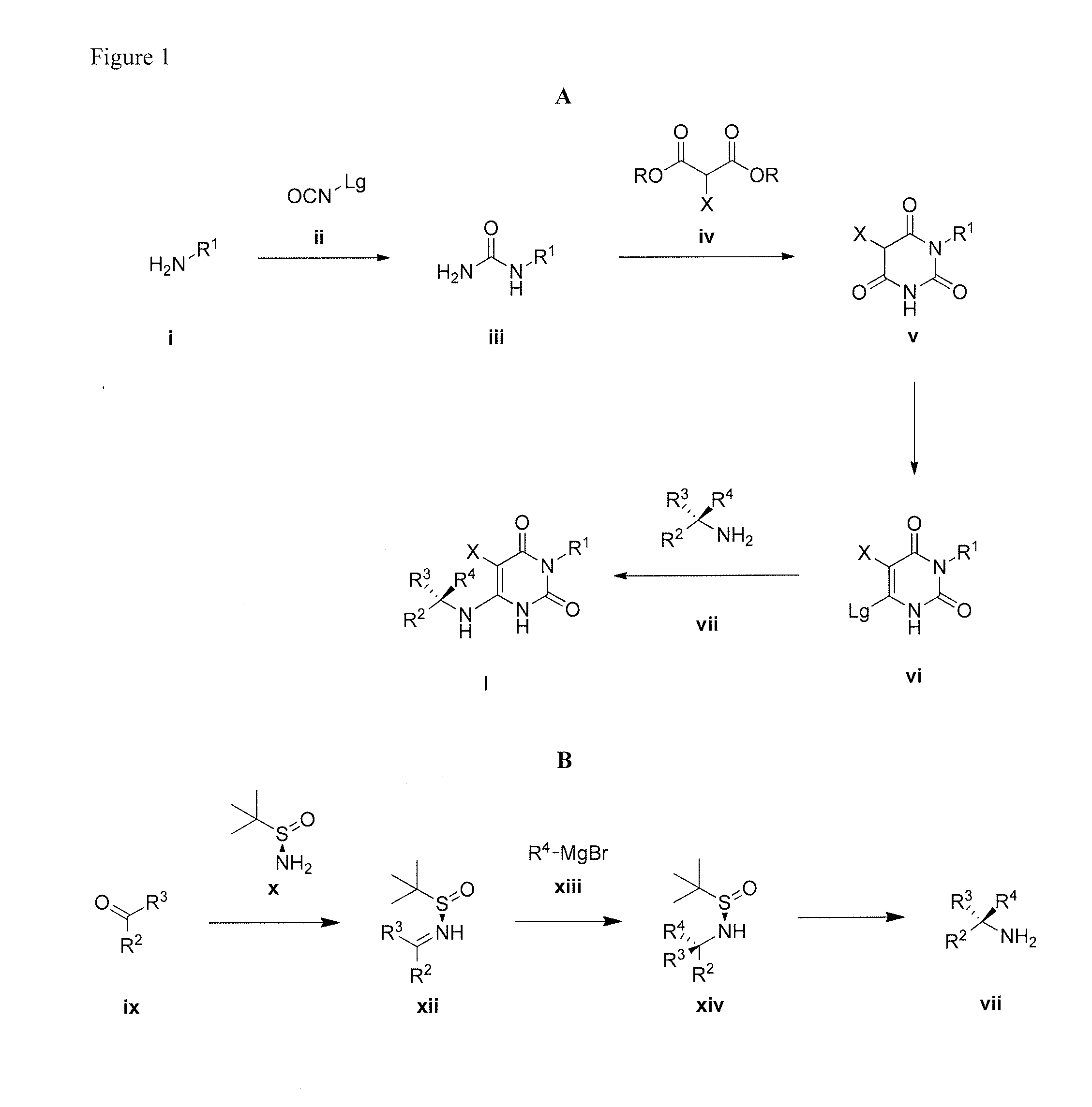
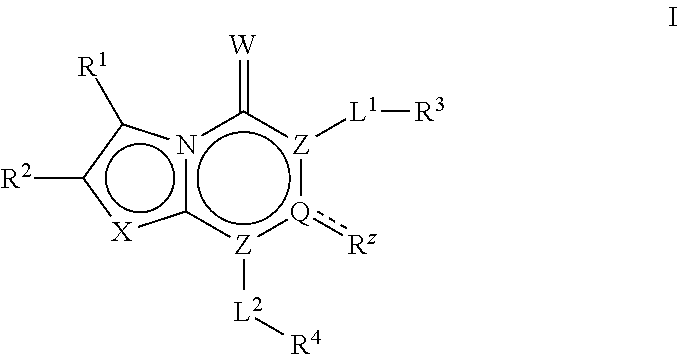
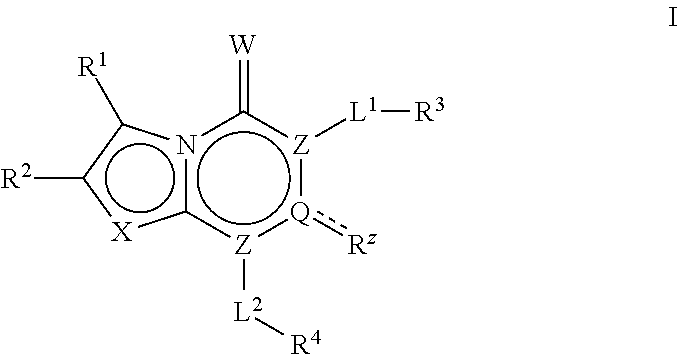
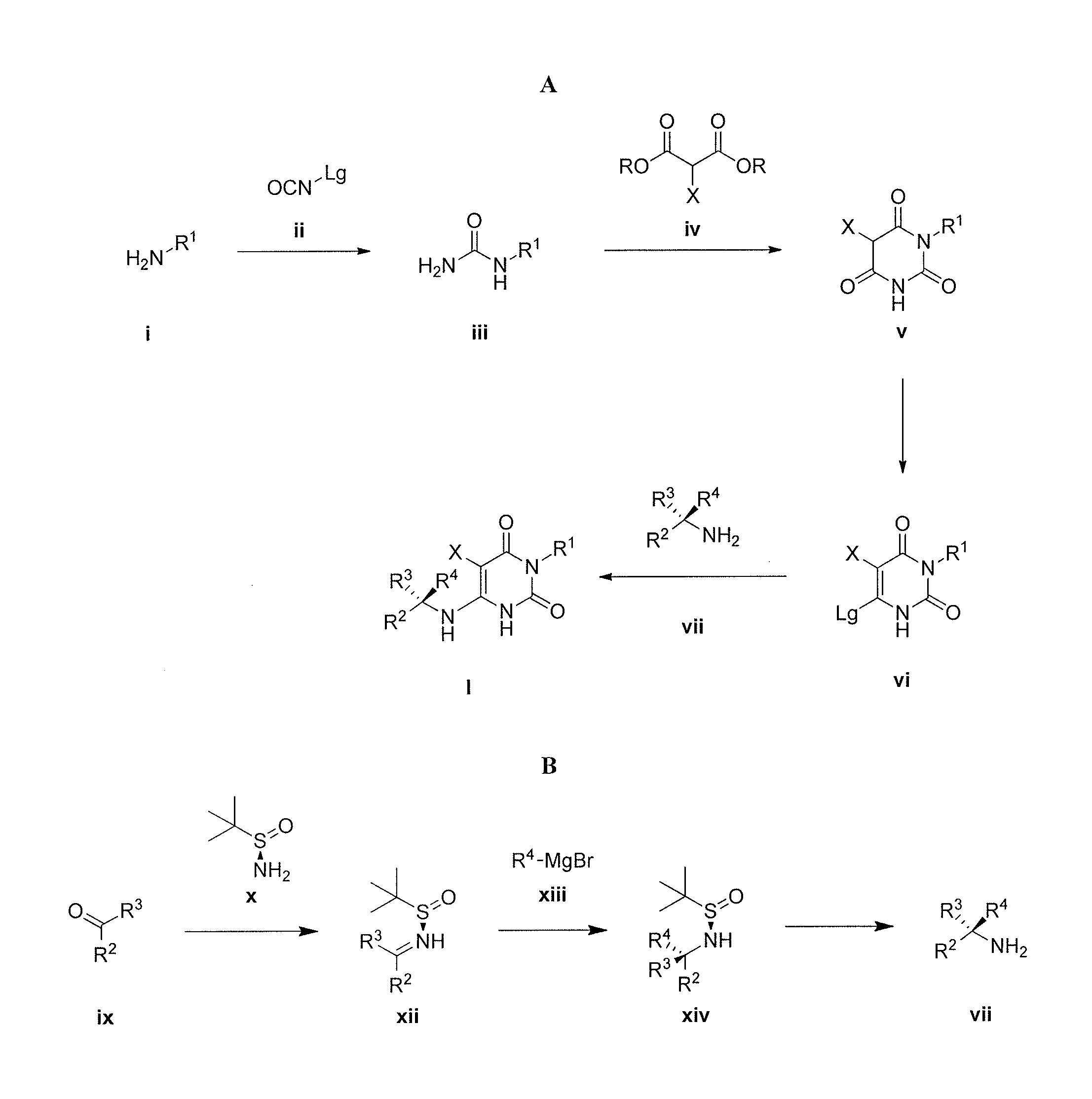
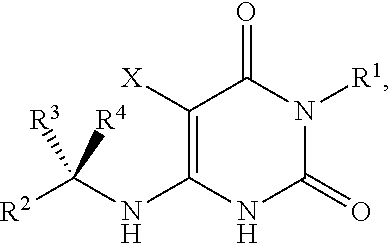
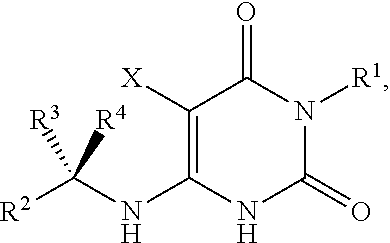
The invention described herein relates to certain pyrimidinedione N-substituted glycine derivatives of formula (I)which are antagonists of HIF prolyl hydroxylases and are useful for treating diseases benefiting from the inhibition of this enzyme, anemia being one example.
Owner:SMITHKLINE BECKMAN CORP
Pyrimidinedione compounds
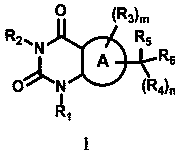
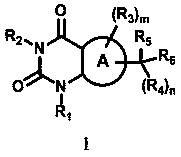
Provided are novel pyrimidine dione compounds and pharmaceutically acceptable salts thereof, that are useful for the treatment of hypertrophic cardiomyopathy (HCM) and conditions associated with left ventricular hypertrophy or diastolic dysfunction. The synthesis and characterization of the compounds and pharmaceutically acceptable salts thereof, are described, as well as methods for treating HCM and other forms of heart disease.
Owner:MYOKARDIA
Cycloalkyl-substituted pyrimidinedione compounds
ActiveUS20140378491A1BiocideOrganic active ingredientsHypertrophic cardiomyopathyLeft ventricular hypertrophy
The present invention provides novel cycloalkyl-substituted pyrimidine dione compounds that are useful for the treatment of hypertrophic cardiomyopathy (HCM) and conditions associated with left ventricular hypertrophy or diastolic dysfunction. The synthesis and characterization of the compounds is described, as well as methods for treating HCM and other forms of heart disease.
Owner:MYOKARDIA
Synthetic method for 5-chloro-6-(2-iminopyrrolidinyl-1-yl)methyl-2,4-(1H,3H)pyrimidinedione hydrochloride
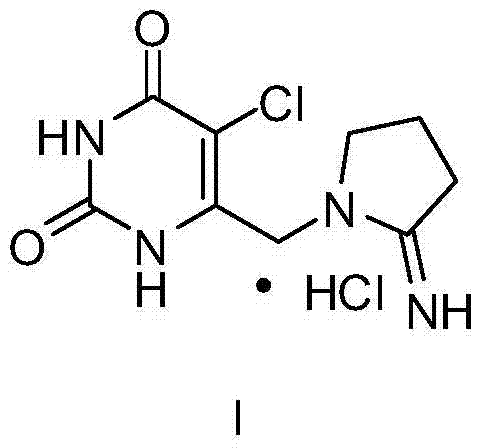
The invention relates to a synthetic method for 5-chloro-6-(2-iminopyrrolidinyl-1-yl)methyl-2,4-(1H,3H)pyrimidinedione hydrochloride. The synthetic method comprises the following steps: with S-methylisothiourea sulfate as a starting raw material, subjecting S-methylisothiourea sulfate and ethyl chloroacetoacetate to a cyclization reaction under an alkaline condition so as to obtain 6-(chloromethyl)-2-(methylthio)pyrimidine-4(3H)-one; carrying out a chlorination reaction on a pyrimidine ring so as to obtain 5-chloro-6-(chloromethyl)-2-(methylthio)pyrimidine-4(3H)-one; then carrying out a hydrolysis reaction under an acidic condition so as to obtain 5-chloro-6-(chloromethyl)pyrimidine-2,4-(1H,3H)-dione; and reacting 5-chloro-6-(chloromethyl)pyrimidine-2,4-(1H,3H)-dione with 2-iminopyrrolidinyl hydrochloride so as to obtain 5-chloro-6-(2-iminopyrrolidinyl-1-yl)methyl-2,4-(1H,3H)pyrimidinedione hydrochloride, i.e., a finished product of hydrochloride. The synthetic method has the advantages of simple operation, a stable intermediate, easy availability, high yield and easy realization of industrial production.
Owner:SHANDONG BESTCOMM PHARMA CO LTD
Acc inhibitors and uses thereof
The present invention provides compounds useful as inhibitors of Acetyl CoA Carboxylase (ACC), compositions thereof, and methods of using the same. Specifically, bicyclic heteroaryl derivatives containing a imidazole, thiazole or oxazole fused to a pyridinone, pyrimidinone or pyrimidindione are provided. These compounds have therapeutic utility toward treating an ACC enzyme mediated disorder such as obesity in a subject, upon administration in an effective amount to said subject.
Owner:NIMBUS DISCOVERY INC
Compound for preparing pyrimidinedione DPP-IV (dipeptidyl peptidase IV) inhibitors
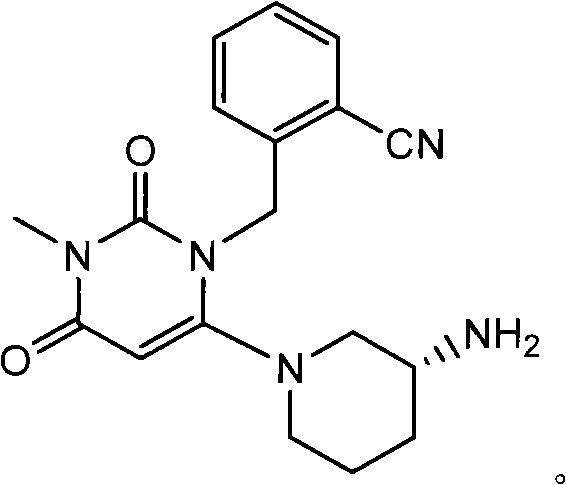
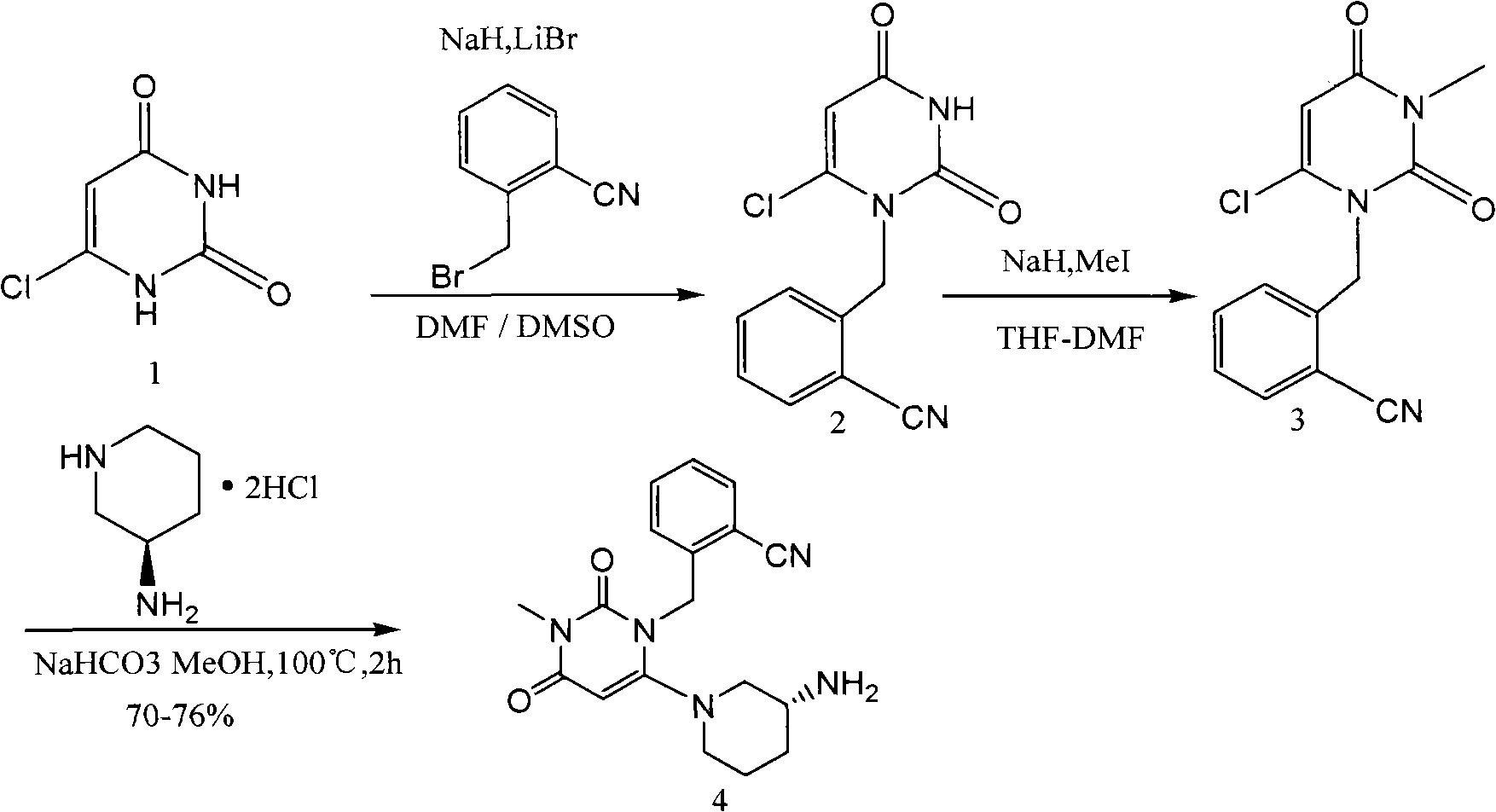
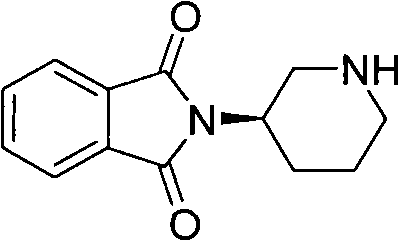
The invention belongs to the technical field of medicines, and in particular relates to a new intermediate shown as a formula I and used for preparing pyrimidinedione DPP-IV (dipeptidyl peptidase IV) inhibitors, such as 2-({6-[(3R)-3-aminopiperidine-1-yl]-3-methyl-2,4-dioxo-3,4-dihydropyrimidine-1(2H)-yl}methyl) cyanophenyl or salts thereof or analogs thereof, and a preparation method of the new intermediate. The formula I is described in the specification.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
Pyrimidinedione compounds
Provided are novel pyrimidine dione compounds and pharmaceutically acceptable salts thereof, that are useful for the treatment of hypertrophic cardiomyopathy (HCM) and conditions associated with left ventricular hypertrophy or diastolic dysfunction. The synthesis and characterization of the compounds and pharmaceutically acceptable salts thereof, are described, as well as methods for treating HCM and other forms of heart disease.
Owner:MYOKARDIA
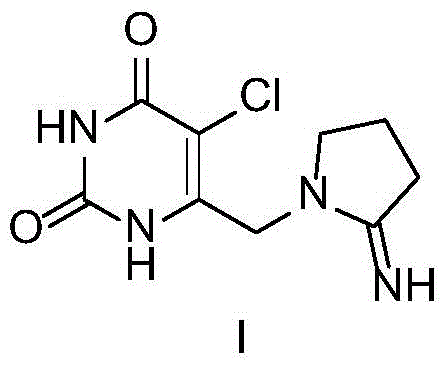
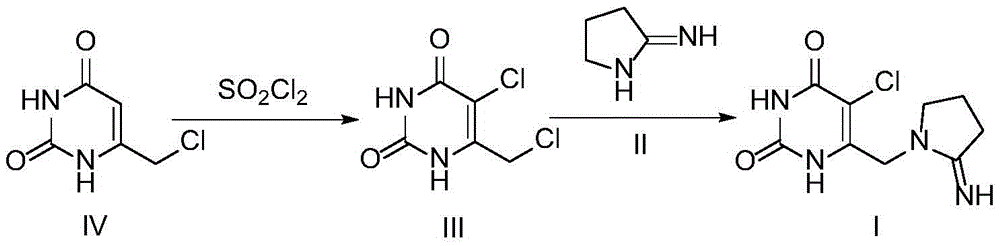
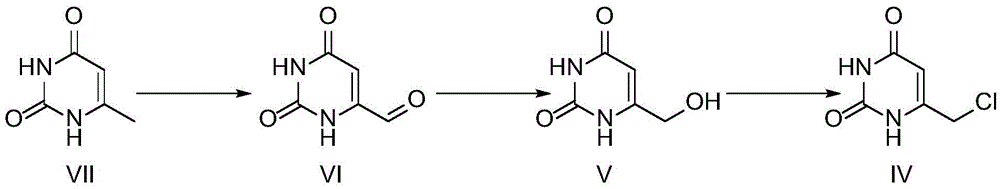
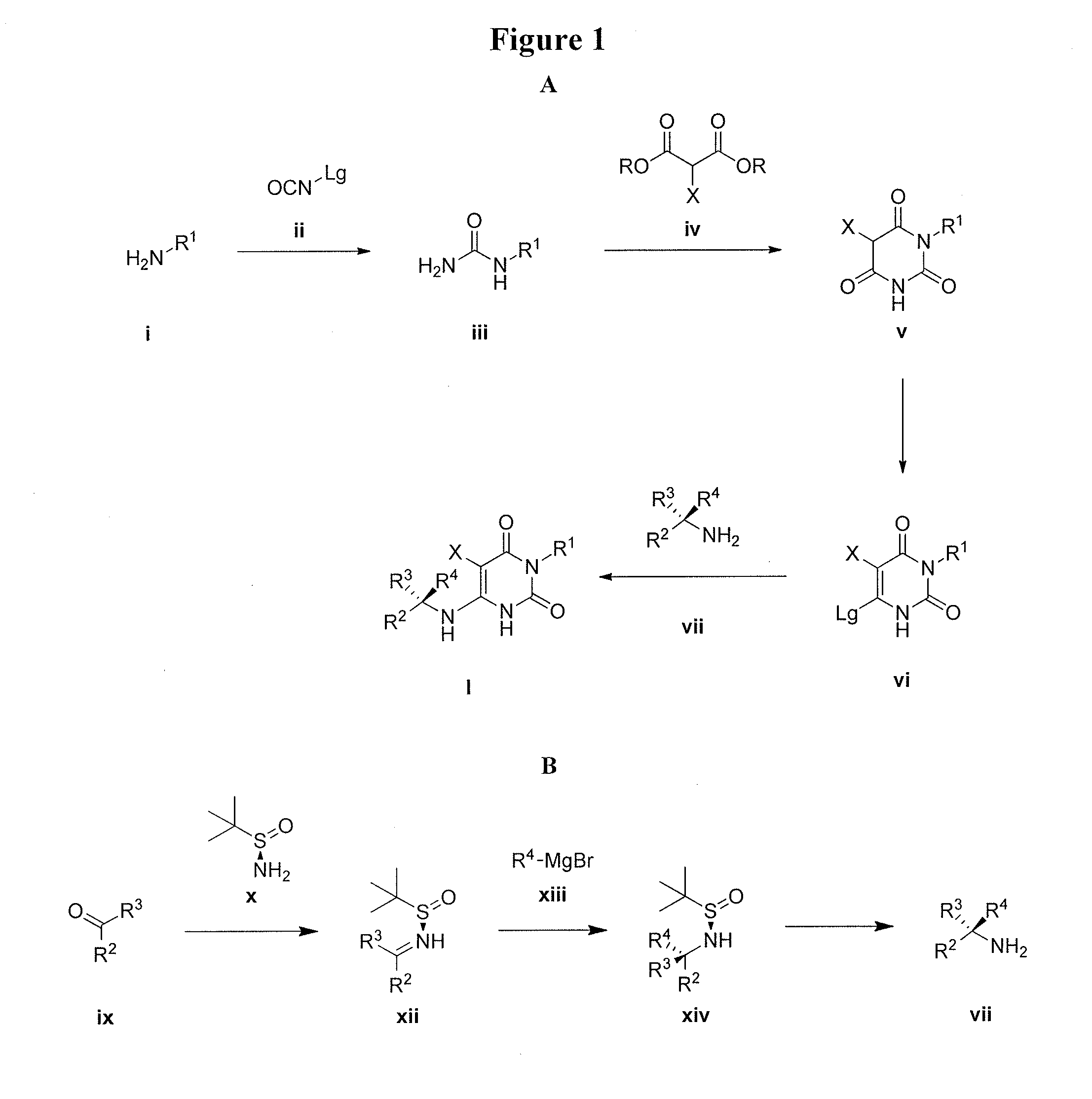
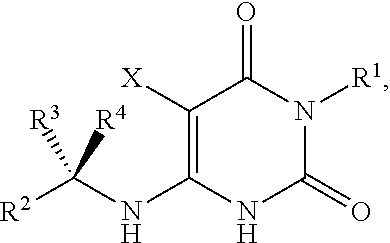
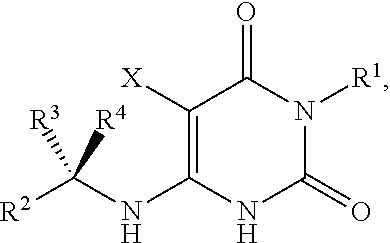
Preparation method of 5-chloro-6-[(2-imino-1-pyrrolidinyl)methyl]-2,4(1H,3H)-pyrimidine dione or salts thereof
The invention provides a preparation method of 5-chloro-6-[(2-imino-1-pyrrolidinyl)methyl]-2,4(1H,3H)-pyrimidine dione or salts thereof, which comprises the following steps: by using 6-methylpyrimidyl-2,4(1H,3H)-dione as an initial raw material, carrying out 6- site methyl oxidation and 5- site hydrogen chlorination, reducing the 6- site formyl group, carrying out condensation with 2-aminopyrrolidine or corresponding salts to obtain the target product. The method is simple to operate and stable in technique, is suitable for industrial production, and has the advantages of high yield, high purity and low cost.
Owner:JIANGSU HANSOH PHARMA CO LTD
ACC inhibitors and uses thereof
Owner:NIMBUS DISCOVERY INC
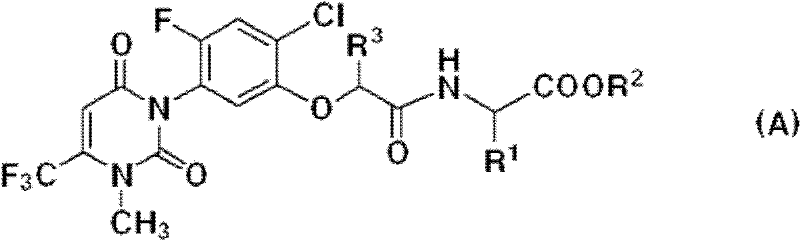
Antiviral 2,4-pyrimidinedione derivatives and process for the preparation thereof
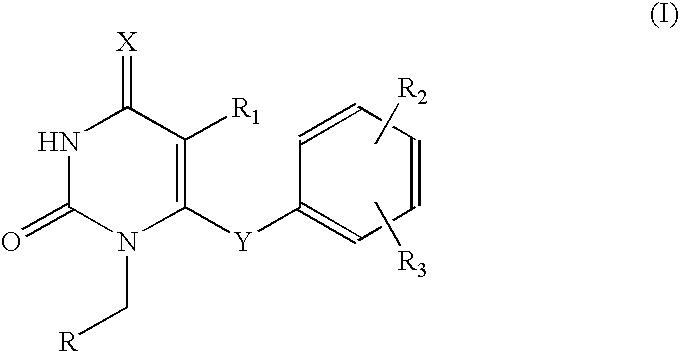
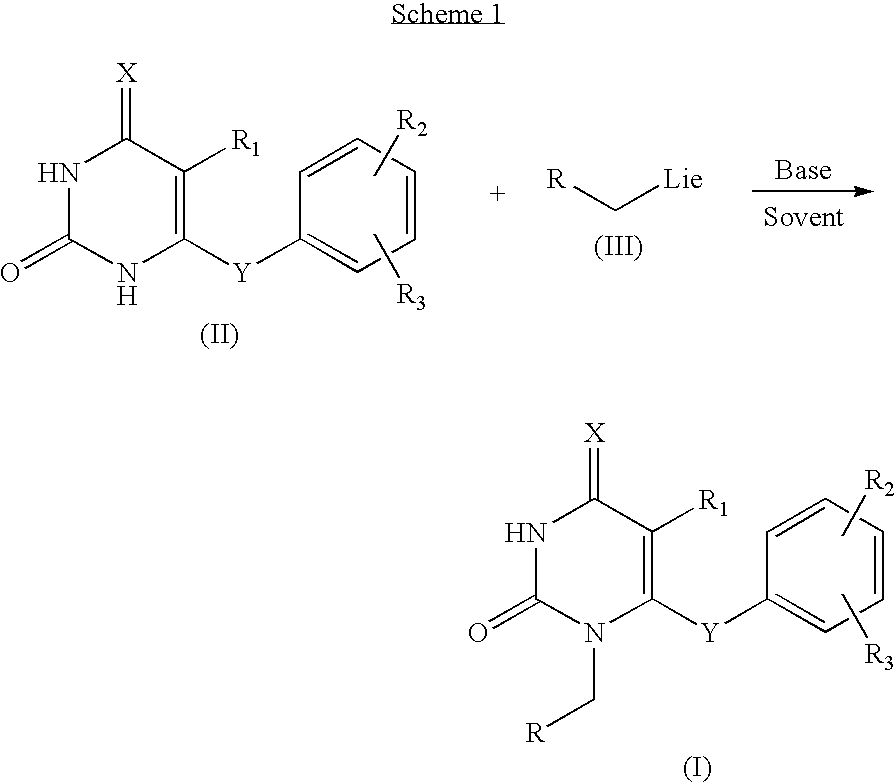
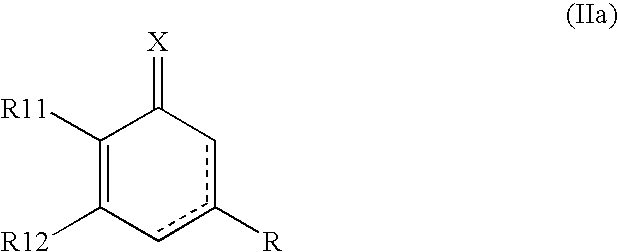
2,4-pyrimidinedione derivatives of formula (I) having high antiviral activity against wild-type and mutant HIV-1 and low toxicity are useful for treating AIDS (I) wherein: R<1 >is a C6-10 aryl or C3-10 heteroaryl group optionally having one or more substituents selected from the group consisting of halogen, C1-6 alkyl, C1-6 alkyl substituted with one or more halogen atoms, C3-4 cycloalkyl, cyano, nitro, hydroxy, thiohydroxy, azido, C1-6 alkoxy, oximino, C1-3 alkyloximino, O-(C1-6 alkyl)-substituted oximino, C-1-6 alkylcarbonyl, C3-6 cycloalkylcarbonyl, hydroxymethyl, azidomethlyl, C1-6 alkoxymethyl, C1-6 acyloxynethyl, carbamoyloxymethyl, anminomethyl, N-(C1-3 alkyl)aminomethyl, N,N-di(C1-3 alkyl)aminomethyl, carboxy, C1-6 alkoxycarbonyl, aziridine, amino, hydroxyethylamino, cyclopropylamino, C1-6 alkylamino, di(C1-6 alkyl)amino, trifluoroacetamido, C1-6 acylamido, carbamoyl, hydroxyethylcarbamoyl, cyclopropylcarbamoyl, C1-6 alkylcarbamoyl, di(C1-6 alkyl)carbamoyl, aminocarbamoyl, dimethylaminocarbamoyl, hydrazino, 1,1-dimethylhydrazino, imidazolyl, triazolyl and tetrazolyl; a tetrahydropyridyl or piperidyl group optionally substituted with a C1-6 alkyl or C1-6 alkoxycarbonyl group; a tetrahydropyranyl group; or a tetrahydrofuryl group; R<2 >is hydrogen, halogen, nitro, cyano, C1-3 alkoxycarbonyl, C1-3 alkylamino, di(C1-3 alkyl)amino, C1-3 alkylcarbamol, di(C1-3 alkyl)carbamoyl, C1-3 alkyl, C3-6 cycloalkyl or benzyl; R<3 >and R<4 >are each independently hydrogen, halogen, hydroxy, cyano, nitro, amino acetamido, trifluoroacetamido, azido, C1-3 alkyl, C-1-3 alkyl substituted with one or more halogen atoms, C1-3 alkoxycarbonyl, carbamoyl C1-3 alkylcarbamoyl, di(C1-3 alkyl) carbamoyl or C-1-3 alkoxy; A is O or S; and Z is O, S, C=O, NH or CH2.
Owner:KOREA RES INST OF CHEM TECH
Antiviral pyrimidinedione derivatives and process for the preparation thereof
The present invention relates to pyrimidinedione derivatives of following formula (I) which are useful as antiviral agents, especially as agents for treatment of AIDS, pharmaceutically acceptable salts thereof, process for the preparation thereof and pharmaceutical compositions containing the same, wherein R represents cyclopropyl; cyclobutyl; cyclohexyl; unsubstituted or mono-, di- or trisubstituted phenyl with a group selected from hydroxy, C1–C4 alkyl, C1–C4 alkoxy, halogen, trifluoromethyl, cyano and amino; 1- or 2-naphthyl; 9-anthracenyl; 2-anthraquinonyl; unsubstituted or substituted pyridyl with a group selected from C1–C4 alkyl, C1–C4 alkoxy, cyano and halogen; 2-, 3- or 4-quinolinyl; oxiranyl; 1-benzotriazolyl; 2-benzoxazolyl; furanyl substituted with C1–C4 alkoxycarbonyl; C1–C4 alkylcarbonyl; or benzoyl, R1 represents halogen or C1–C4 alkyl, R2 and R3 represent independently hydrogen or C1–C4 alkyl, X represents oxygen atom, and Y represents oxygen atom, sulfur atom or carbonyl.
Owner:SAMJIN PHARMA
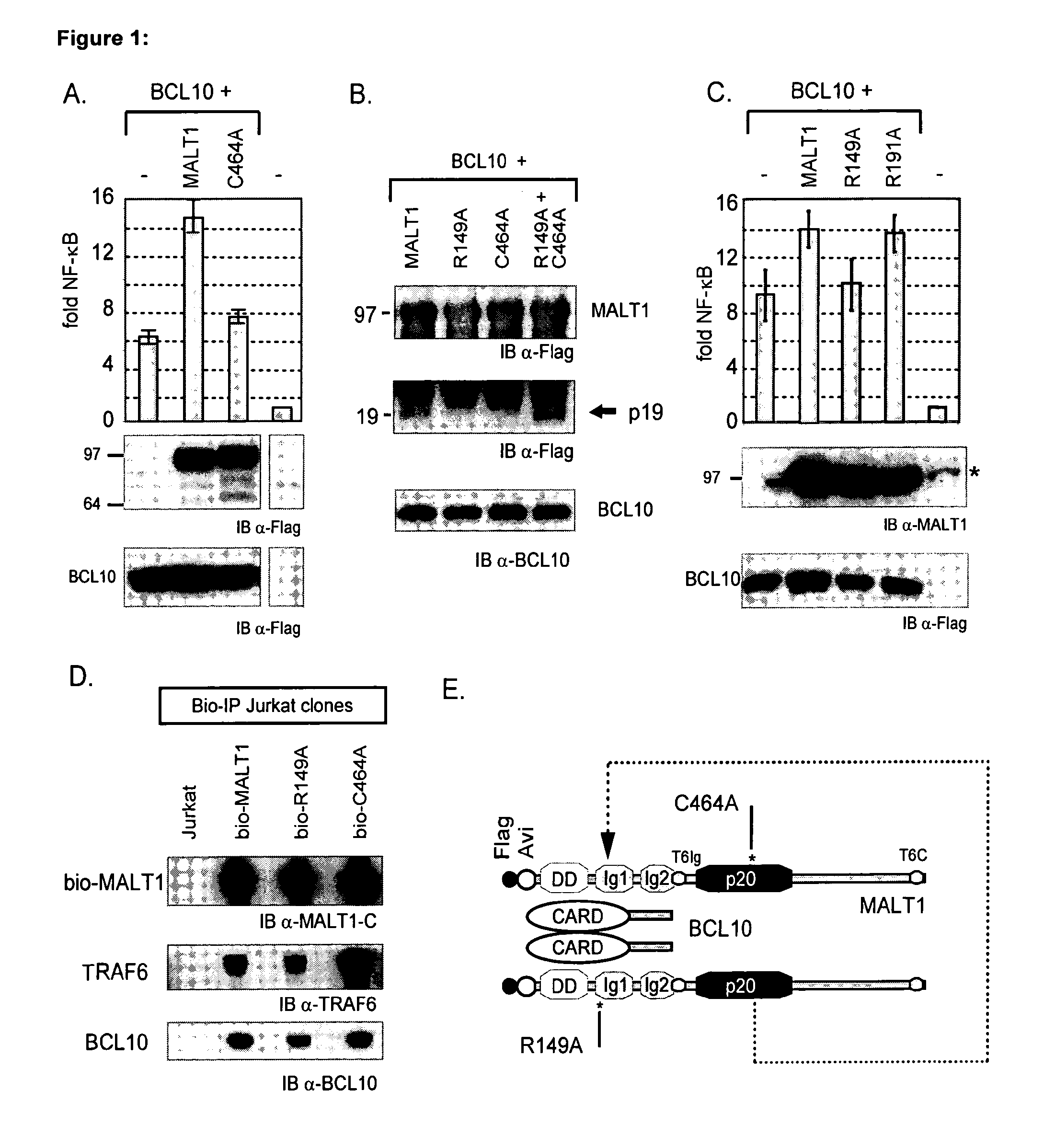
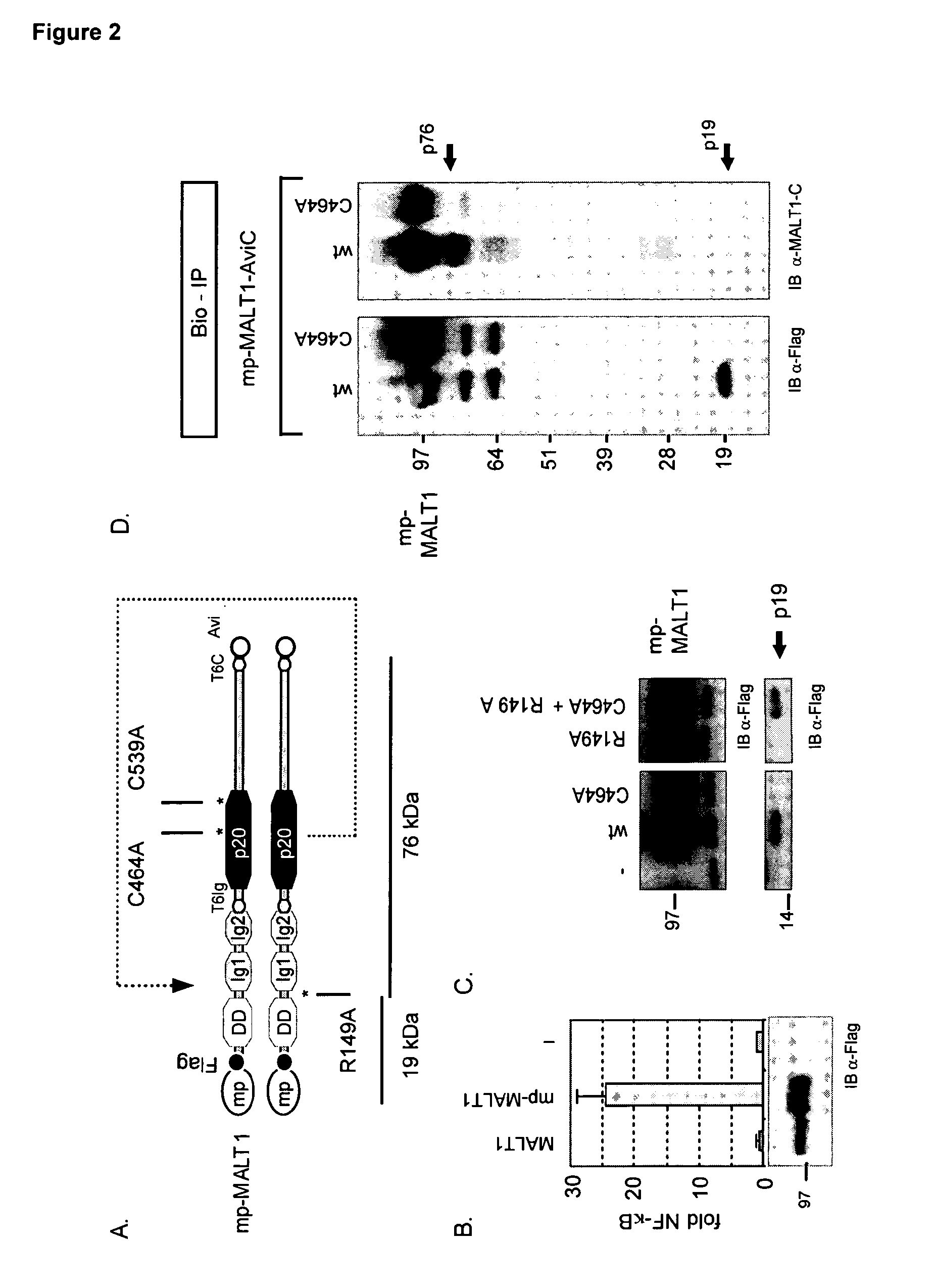
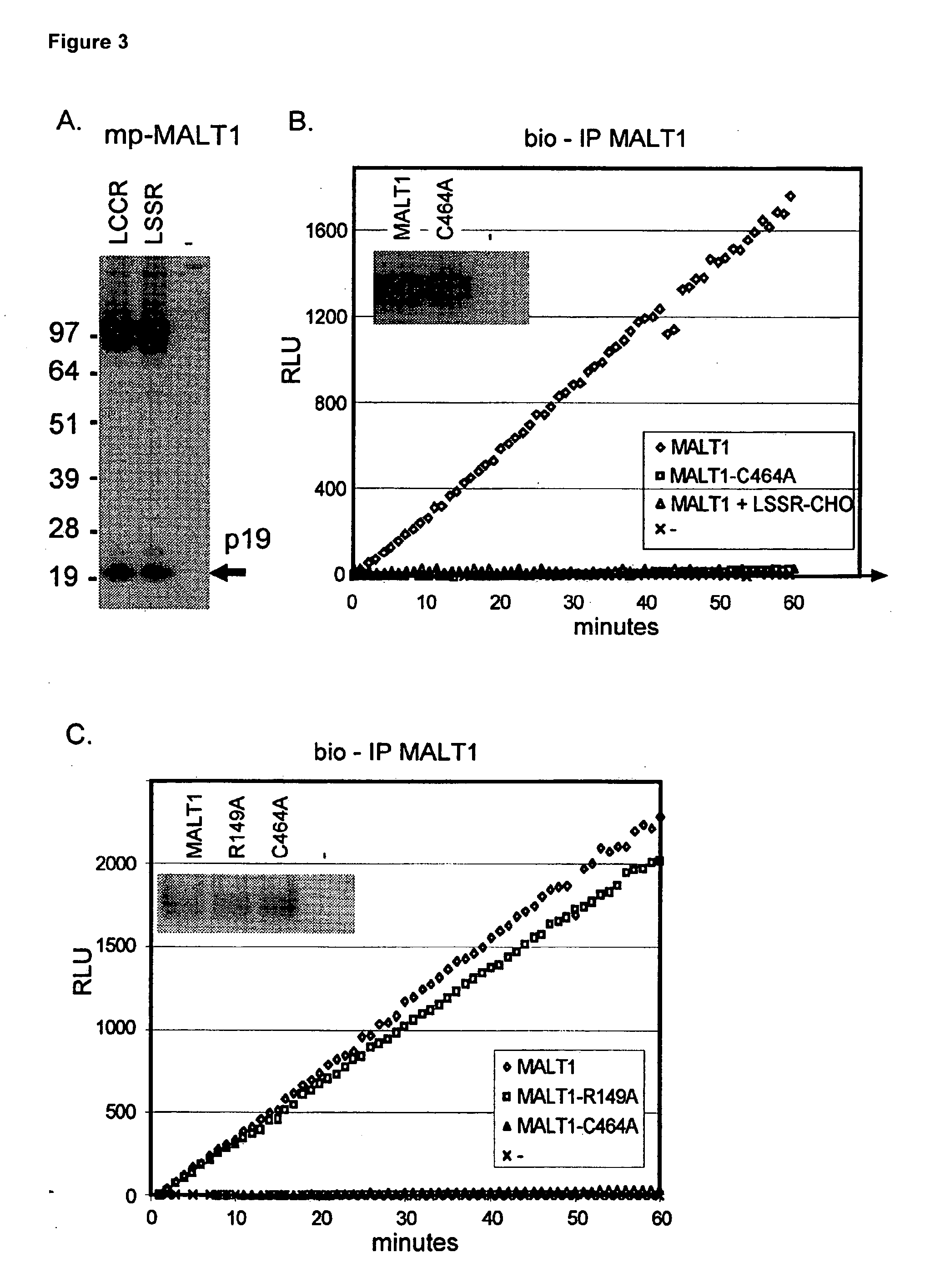
Inhibitors of malt1 proteolytic activity and uses thereof
The present invention relates to inhibitors of MALT1 proteolytic and / or autoproteolytic activity. More specifically, it relates to compounds such as, but not limited to peptide derivates such as Z-LSSR-CHO (see SEQ ID NO:1), Z-LSSR-CMK (see SEQ ID NO:1), Z-GASR-CHO (see SEQ ID NO:2), and Z-GASR-CMK (see SEQ ID NO:2), and small compounds such as 5-{[5-(3-chloro-4-methylphenyl)-2-furyl]methylene}-2-thioxodihydro-4,6(1H,5H)-pyrimidinedione and variants thereof, and the use of those compounds for the preparation of a medicament. The invention relates further to a method to screen for inhibitors of the MALT1 proteolytic and / or autoproteolytic activity.
Owner:VLAAMS INTERUNIVERSITAIR INST VOOR BIOTECHNOLOGIE VZW +2
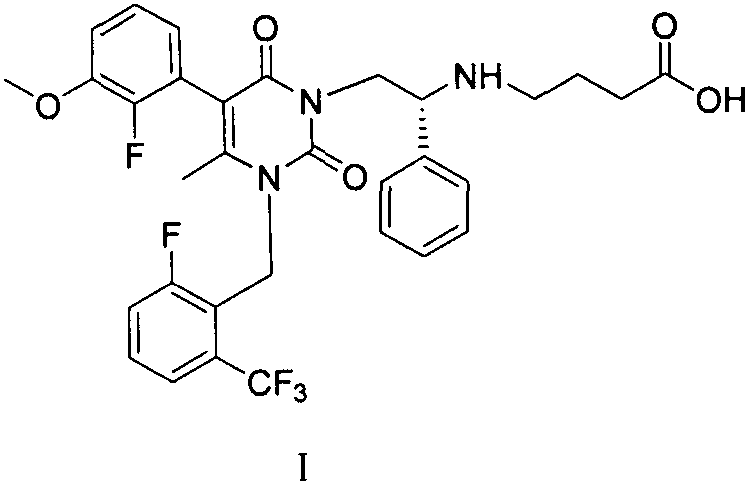
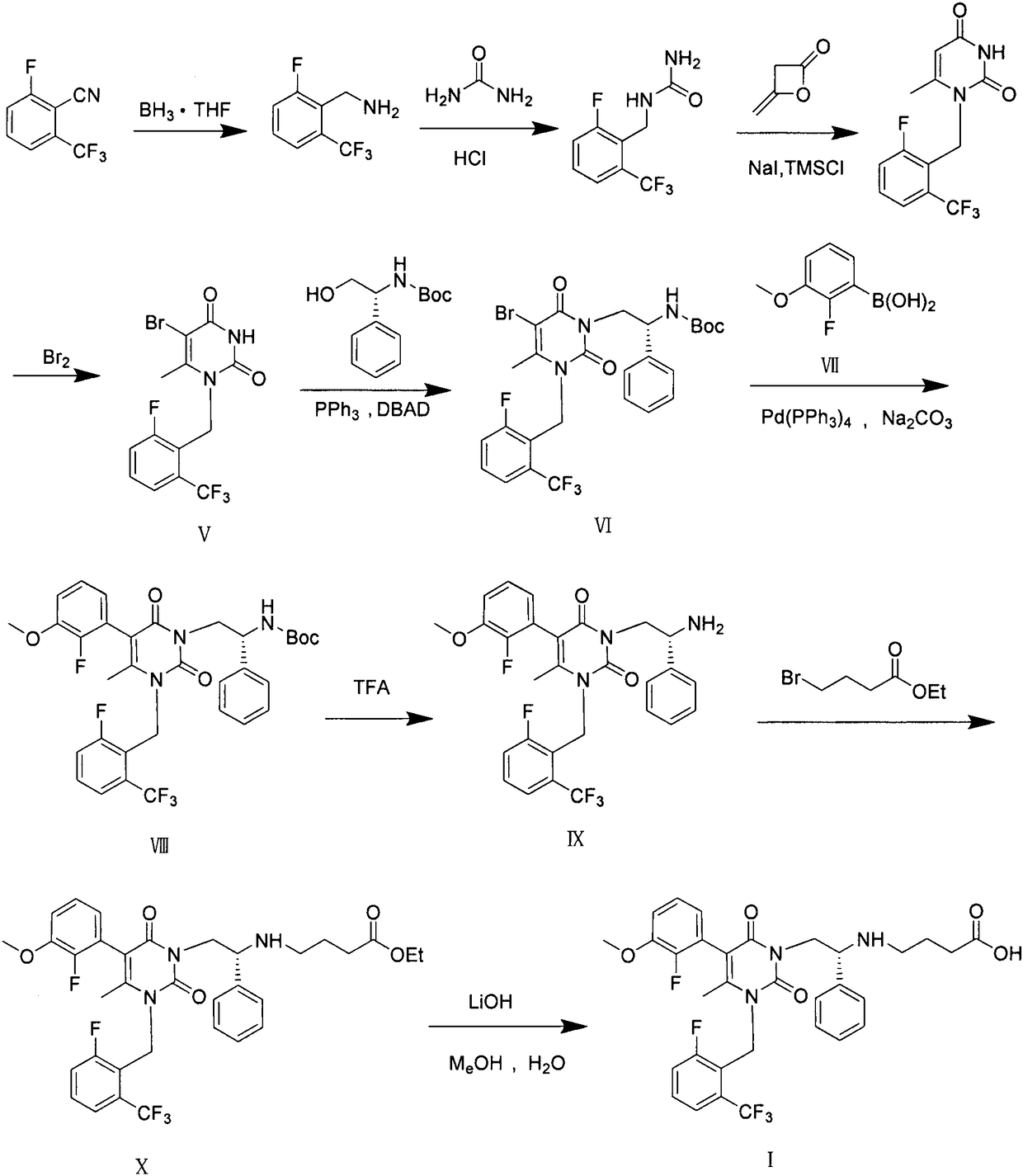
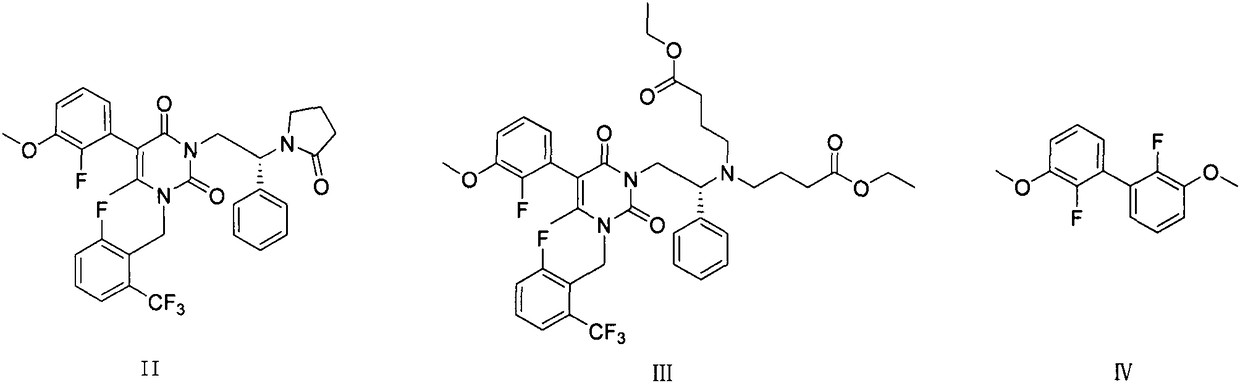
Preparation method of elagolix impurities
The invention relates to a synthetic method of three elagolix impurities which is important to the synthesis of high-quality elagolix. The invention mainly focuses on the study on a preparation methodof (R)-5-(2-fluoro-3-methoxyphenyl)-1-[2-fluoro-6-(trifluoromethyl)benzyl]-6-methyl-3-[2-(2-oxopyrrolidine-1-yl)-2-phenylethyl]pyrimidin-2,4(1H,3H)-dione (II), diethyl (R)-4,4'-[[2-[5-(2-fluoro-3-methoxyphenyl)-3-[2-fluoro-6-(trifluoromethyl)benzyl]-4-methyl-2,6-dioxo-3,6-dihydropyrimidin-1(2H)-yl]-1-phenylethyl]azadiyl]-dibutyrate (III), and 2,2'-difluoro-3,3'-dimethyoxy-1,1'-biphenyl (IV). A synthesis route is shown in the description.
Owner:CHINA PHARM UNIV
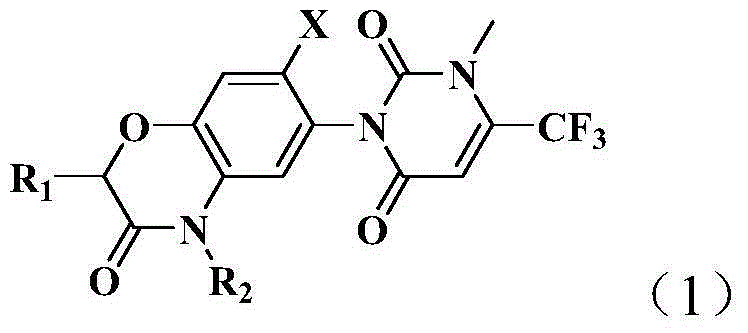
Pyrimidine diketone compounds containing benzoxazine ring and application thereof
ActiveCN105272973AImprove herbicidal activityOrganic chemistryHerbicides and algicidesDiketoneHydrogen
The invention discloses a kind of pyrimidine diketone compounds containing a benzoxazine ring and application thereof. The pyrimidine diketone compounds containing the benzoxazine ring are compounds with the structure shown as a general formula (1) in the specification, and in the formula (1), R1 is selected from hydrogen or an alkyl with the carbon atom number of 1-6, R2 is an ester with the carbon atom number of 2-10, and X is selected from halogen. The pyrimidine diketone compounds containing the benzoxazine ring possess high weeding activity.
Owner:HUAZHONG NORMAL UNIV
Antiviral 2,4-pyrimidinedione derivatives and process for the preparation thereof
PCT No. PCT / KR97 / 00084 Sec. 371 Date Nov. 10, 1998 Sec. 102(e) Date Nov. 10, 1998 PCT Filed May 15, 1997 PCT Pub. No. WO97 / 43266 PCT Pub. Date Nov. 20, 19976-aryloxy and 6-arylcarbonyl 2,4-pyrimidinedione derivatives of the formula(I) having high antiviral activity against HIV-1 and low toxicity are useful for treating AIDS: wherein: R1 is hydrogen or a C1-10 alkyl group optionally having a substituent selected from the group consisting of aryl, hydroxy, C1-10 alkoxy and C2-5 alkylcarbonyloxy groups; R2 is hydrogen or a C1-10 alkyl group optionally having an aryl substituent; R3 and R4 are each hydrogen or a C1-3 alkyl group; and A is oxygen or a carbonyl group.
Owner:KOREA RES INST OF CHEM TECH
Prolyl Hydroxylase Inhibitors
The invention described herein relates to certain pyrimidinedione N-substituted glycine derivatives of formula (I)which are antagonists of HIF prolyl hydroxylases and are useful for treating diseases benefiting from the inhibition of this enzyme, anemia being one example.
Owner:GLAXO SMITHKLINE LLC
Pyrimidinediones as tyrosine kinase inhibitors
The present invention relates to a compound of formula (I)or its stereoisomers, tautomers, solvates, hydrates, prodrugs, pharmaceutically acceptable salts or mixtures thereof, or pharmaceutical composition comprising a compound of formula (I) or a pharmaceutically acceptable salt thereof. The present invention also provides a method for the prophylaxis or treatment of a medical condition associated with protein kinase, by administering a pharmaceutically effective amount of the compound of formula (I) or salts thereof.
Owner:JUBILANT BIOSYS LTD
Preparation method of tipiracil intermediate
The invention provides a preparation method of a tipiracil intermediate which is 6-(chloromethyl)-2, 4-(1H, 3H)-pyrimidinedione. 6-methyl-2, 4-(1H, 3H)-pyrimidinedione as an initial raw material undergoes an iodine replacement reaction at the 5th site of a compound and then the product undergoes reduction and chlorination reactions at the 6th site to produce a desired product. The preparation method has the advantages of stable processes, high yield and low cost and is suitable for industrial production.
Owner:HARVEST PHARMA HUNAN CO LTD
Bicyclic-pyrimidinedione compounds
ActiveUS20160176868A1FavorablyBiocideOrganic chemistryHypertrophic cardiomyopathyLeft ventricular hypertrophy
The present invention provides novel bicyclic pyrimidinedione compounds that are useful for the treatment of hypertrophic cardiomyopathy (HCM) and conditions associated with left ventricular hypertrophy or diastolic dysfunction. The synthesis and characterization of the compounds is described, as well as methods for treating HCM and other forms of heart disease.
Owner:MYOKARDIA
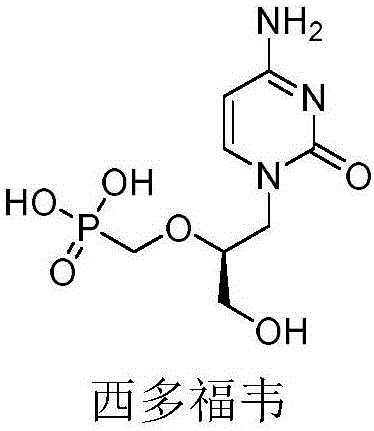
2′-chloro aminopyrimidinone and pyrimidine dione nucleosides
Provided herein are formulations, methods and substituted 2′-chloro aminopyrimidinone and pyrimidine dione compounds of Formula (I) for treating Pneumovirinae virus infections, including respiratory syncytial virus infections, as well as methods and intermediates for synthesis of substituted 2′-chloro aminopyrimidinone and pyrimidine dione compounds.
Owner:GILEAD SCI INC
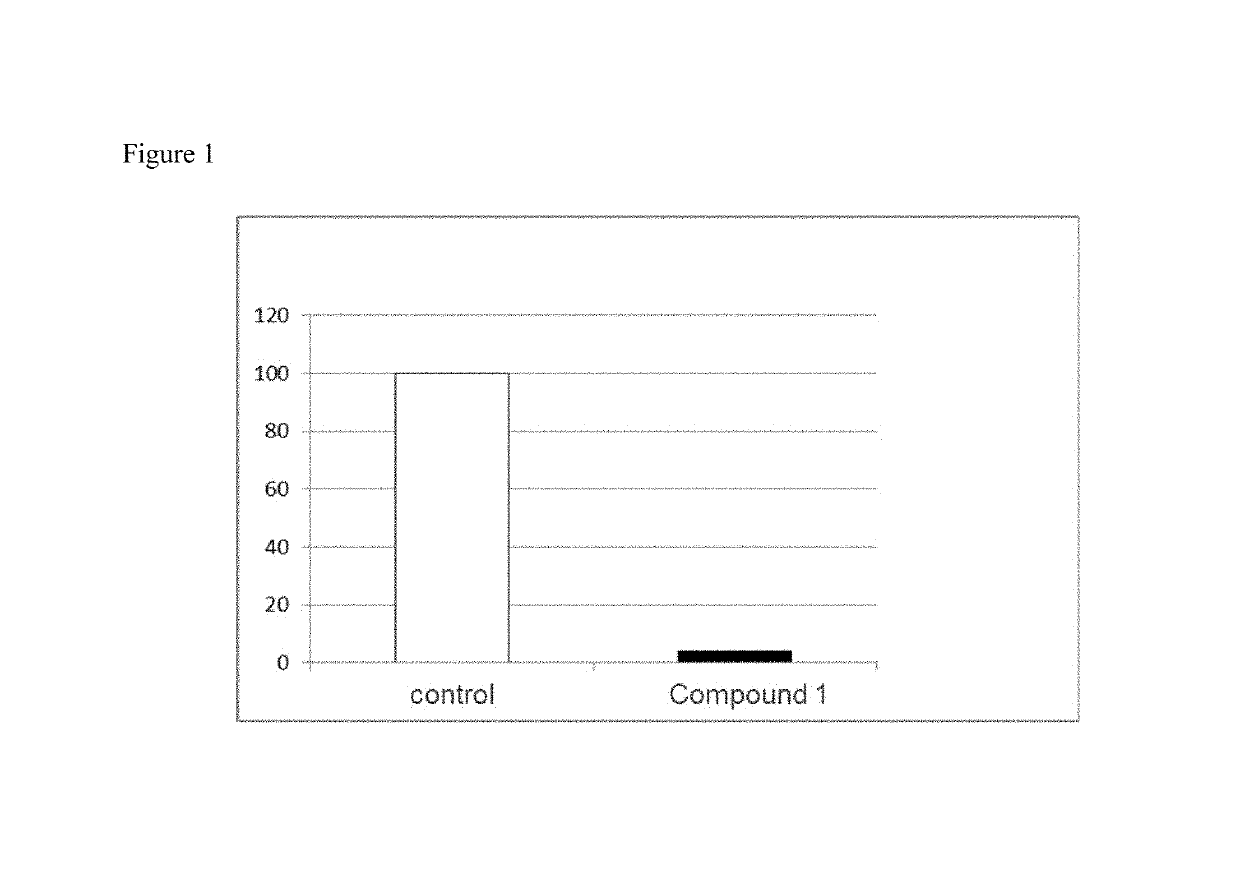
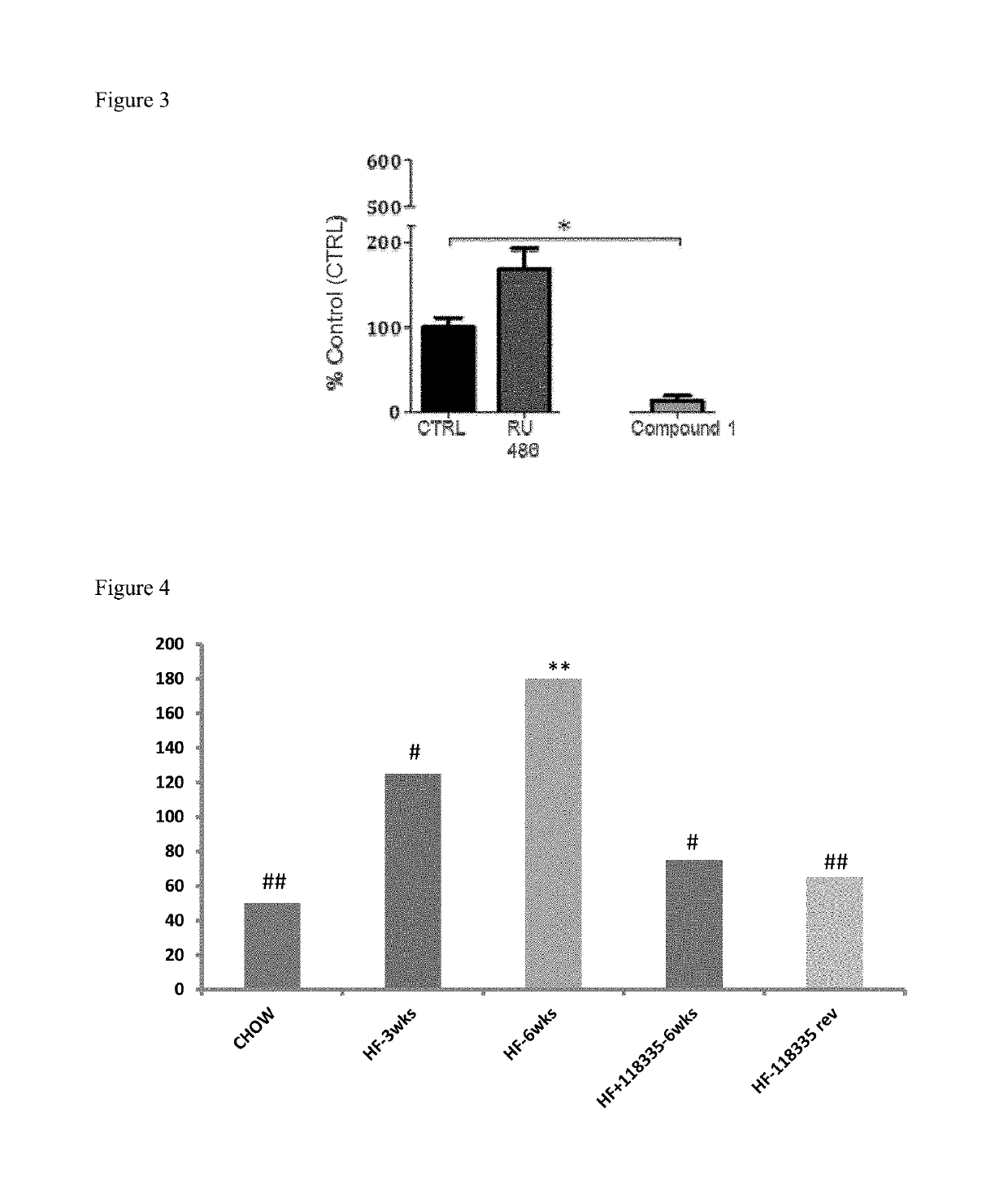
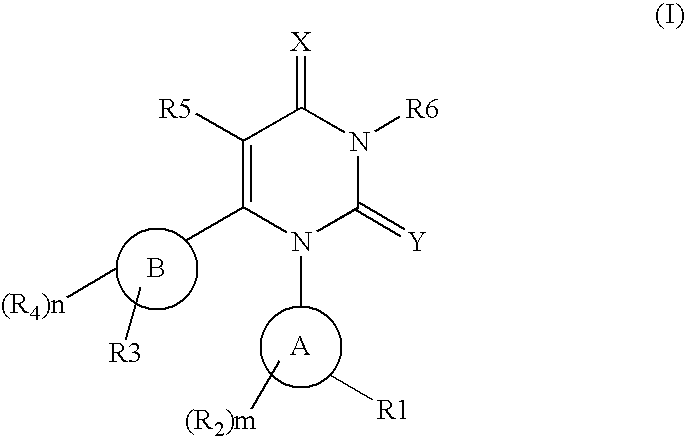
Fatty liver disease treatment using glucocorticoid and mineralocorticoid receptor antagonists
The present invention provides treatment of fatty liver disease using a class of pyrimidinedione cyclohexyl compounds.
Owner:CORCEPT THERAPEUTICS INC
Novel pyrimidinedione derivatives
Pyrimidinedione derivatives of the general formula (I), their derivatives, analogs, tautomeric forms, stereoisomers, polymorphs, hydrates, solvates, pharmaceutically acceptable salts and pharmaceutically acceptable compositions containing them are useful for the treatment of inflammation and immunological diseases.
Owner:ORCHID RES LAB
Novel crystal of pyrimidinedione compound hydrochloride and preparation method thereof
InactiveCN104744443ASuitable for developing applicationsImprove stabilityOrganic chemistry methodsX-rayPyrrolidine
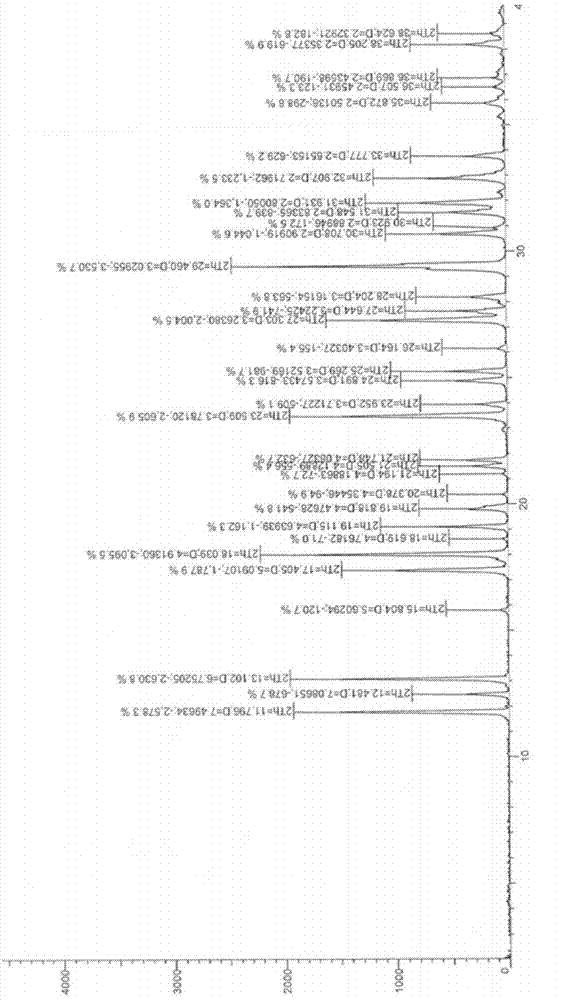
The invention relates to a novel crystal of pyrimidinedione compound hydrochloride and a preparation method thereof. The invention discloses the novel crystal of 5-chloro-6-[(2-imino-1-pyrrolidyl)methyl]-2,4(1H, 3H)-pyrimidinedione hydrochloride shown in the formula I and the preparation method thereof. The novel crystal can produce X-ray diffraction peaks at diffraction angles 2 theta of 11.73 degrees, 13.03 degrees, 17.33 degrees, 17.97 degrees, 23.45 degrees, 27.23 degrees, 29.40 degrees and 32.85 degrees, has good stability and reappearance and is suitable for medicine development and application.
Owner:JIANGSU HANSOH PHARMA CO LTD
Prolyl hydroxylase inhibitors
The invention described herein relates to certain pyrimidinedione N-substituted glycine derivatives of formula (I)which are antagonists of HIF prolyl hydroxylases and are useful for treating diseases benefiting from the inhibition of this enzyme, anemia being one example.
Owner:GLAXO SMITHKLINE LLC
Novel crystal form of Tipracil hydrochloride and preparation method thereof
The invention discloses a new crystal form of Tipracil hydrochloride and a preparation method thereof. Particularly, the invention relates to a crystal form of Tipracil hydrochloride shown in the formula (1), namely 5-chloro-6-[(2-imino-pyrrolidine) methyl]-2,4(1H,3H)-pyrimidinedione hydrochloride, and a preparation method of the new crystal form of Tipracil hydrochloride. The crystal form is stable and easily prepared and meets the application requirement of medicine preparations. The formula (1) is shown in the specification.
Owner:JIANGSU HANSOH PHARMA CO LTD
Uracil-based compounds, and herbicides comprising same
ActiveCN102203071ALow herbicidal activityImprove herbicidal activityBiocideOrganic chemistryBiotechnologyUracil
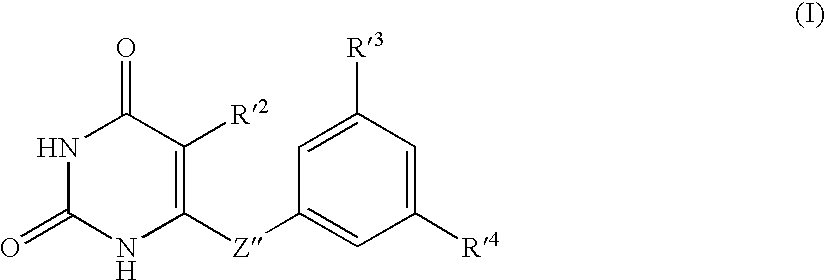
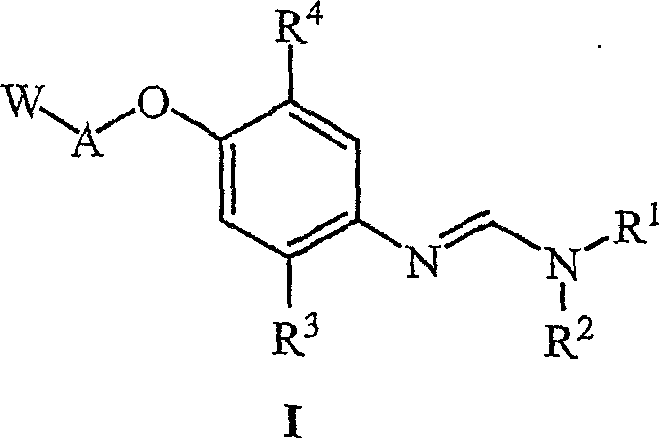
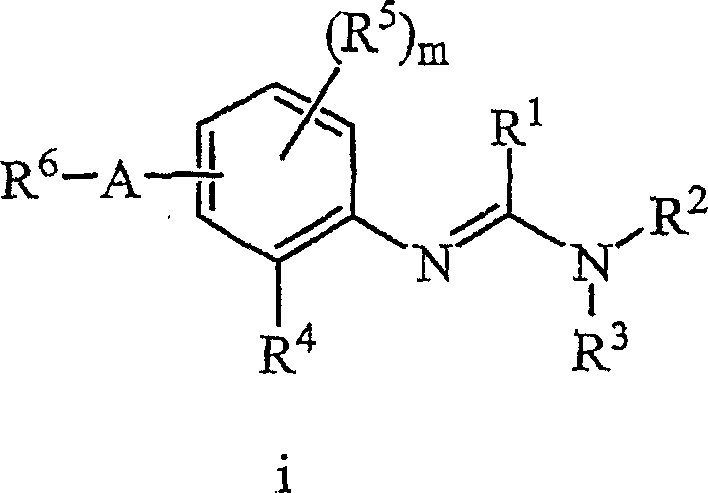
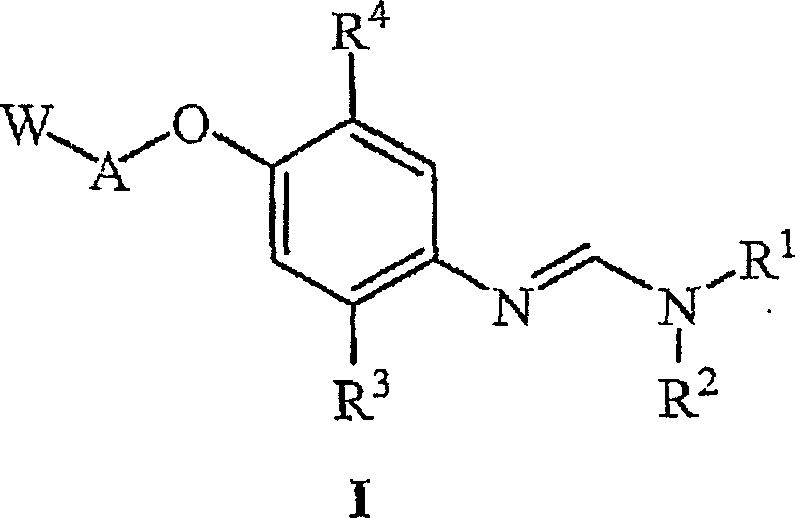
The present invention relates to uracil-based compounds, to a method for producing the compounds, and to herbicides comprising the compounds as active ingredients. R1, R2, R3, R4, R5, X, Y, Z, and W are as defined in the detailed description of the invention. [Keywords] uracil, herbicides, pyrimidinedione.
Owner:FARMHANNONG CO LTD
N-hydroxyl heterocycle pyrimindine diketone derivative and preparation method and application thereof
The invention discloses a quinazoline ketone derivative and a preparation method and application thereof.The compound is of the structure as shown in the general formula (I).The invention further relates to a drug composition containing the compound in the formula (I).Activity screening experiments show that the compound has good anti-acne-virus and anti-adenovirus activity, and therefore application of the compound to preparing anti-acne-virus and anti-adenovirus drugs is also provided.Please see the formula (I) in the description.
Owner:SHANDONG UNIV
Pyrimidinedione derivative capable of inhibiting monocarboxylate transporter
ActiveCN109422749AAltered pharmacodynamic responseChange mechanical propertiesOrganic active ingredientsNervous disorderMonocarboxylate transporterChemical compound
The invention relates to a pyrimidinedione derivative capable of inhibiting a monocarboxylate transporter, the pyrimidinedione derivative is a formula (I) compound and / or a pharmaceutically acceptablesalt thereof, and / or a stereoisomer thereof, and / or a solvate thereof, the compound has the effect of inhibiting the activity of the monocarboxylate transporter, and also comprises a pharmaceutical composition containing the formula (I) compound and the use of the pharmaceutical composition in treatment.
Owner:CHONGQING PHARMA RES INST
Fungicidal mixtures of amidinylphenyl compounds
Disclosed are fungicidal mixtures, compositions and methods for controlling plant diseases relating to combinations comprising (a) at least one compound selected from phenylamidines of Formula I, N-oxides, and agriculturally suitable salts thereof (I) wherein A is C3alkylene, optionally substituted with one or two methyl; W is CR<5>R<6>R<7>or SiR<8>R<9>R<10>; and R<1>, R<2>, R<3>, R<4>, R<5>, R<6>, R<7>, R<8>, R<9> and R<10>are as defined in the disclosure; and (b) at least one compound selected from alkylenebis(dithiocarbamate) fungicides, compounds acting at the bc1complex of the fungal mitochondrial respiratory electron transfer site, cymoxanil, compounds acting at the demethylase enzyme of the sterol biosynthesis pathway, morpholine and piperidine compounds that act on the sterol biosynthesis pathway, phenylamide fungicides, pyrimidinone fungicides, chlorothalonil, carboxamides acting at complex II of the fungal mitochondrial respiratory electron transfer site, quinoxyfen, metrafenone, cyflufenamid, cyprodinil, copper compounds, phthalimide fungicides, fosetyl-aluminum, benzimidazole fungicides, cyazofamid, fluazinam, iprovalicarb, propamocarb, validamycin, dichlorophenyl dicarboximide fungicides, zoxamide and dimethomorph, and their agriculturally suitable salts.
Owner:EI DU PONT DE NEMOURS & CO
Oral administrable pharmaceutical composition
InactiveUS20140356431A1Secured formulation stabilityStable storageBiocideCarbohydrate active ingredientsHigh humidityBULK ACTIVE INGREDIENT
The present invention provides an FTD and TPI-containing orally administrable pharmaceutical composition which can be orally administered and is stable even under high-humidity conditions.An orally administrable pharmaceutical composition which comprises α,α,α-trifluorothymidine and 5-chloro-6-(2-iminopyrrolidine-1-yl)methyl-2,4(1H,3H)-pyrimidine dione hydrochloride as active ingredients and additives having a critical relative humidity of 85% or more at 25° C. as an excipient.
Owner:TAIHO PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Preparation method of 5-chloro-6-[(2-imino-1-pyrrolidinyl)methyl]-2,4(1H,3H)-pyrimidine dione or salts thereof Preparation method of 5-chloro-6-[(2-imino-1-pyrrolidinyl)methyl]-2,4(1H,3H)-pyrimidine dione or salts thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/4daf6343-d935-4bb0-9376-22f58276f0ed/BDA0000481599190000011.PNG)
![Preparation method of 5-chloro-6-[(2-imino-1-pyrrolidinyl)methyl]-2,4(1H,3H)-pyrimidine dione or salts thereof Preparation method of 5-chloro-6-[(2-imino-1-pyrrolidinyl)methyl]-2,4(1H,3H)-pyrimidine dione or salts thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/4daf6343-d935-4bb0-9376-22f58276f0ed/BDA0000481599190000031.PNG)
![Preparation method of 5-chloro-6-[(2-imino-1-pyrrolidinyl)methyl]-2,4(1H,3H)-pyrimidine dione or salts thereof Preparation method of 5-chloro-6-[(2-imino-1-pyrrolidinyl)methyl]-2,4(1H,3H)-pyrimidine dione or salts thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/4daf6343-d935-4bb0-9376-22f58276f0ed/BDA0000481599190000041.PNG)