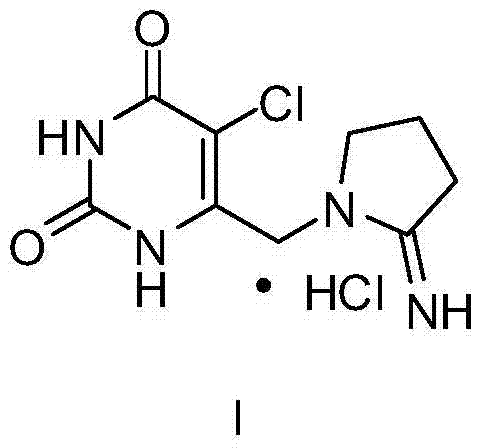
Novel crystal of pyrimidinedione compound hydrochloride and preparation method thereof
A kind of technology of compound and crystal form, applied in the field of pyrimidinedione compound hydrochloride
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1: Preparation of Form I of Tipiracil Hydrochloride Using Dilute Hydrochloric Acid
[0021] Compound 1 (50g, 0.18mol) was added to purified water (250L), heated to 70°C to dissolve, then 25mL of concentrated hydrochloric acid was added dropwise, kept stirring and crystallized for 2h, then cooled down to room temperature for 2h. After filtering, the resulting solid was air-dried at 40°C until constant weight. The target product (42.3 g, white solid) was obtained with a yield of 84.6%.
Embodiment 2
[0022] Embodiment 2: Preparation of crystalline form I of Tipiracil hydrochloride using hydrochloric acid ethanol
[0023] Compound 1 (50g, 0.18mol) was added to purified water (250L), heated to 70°C to dissolve, then 25mL of concentrated hydrochloric acid was added dropwise, stirred and crystallized for 2h, 1000mL of ethanol was added, and the temperature was naturally cooled to room temperature for 2h. After filtering, the resulting solid was air-dried at 40°C until constant weight. The target product (46.5 g, white solid) was obtained with a yield of 93.0%.
experiment example 1
[0024] Experimental example 1: Influencing factor experiment
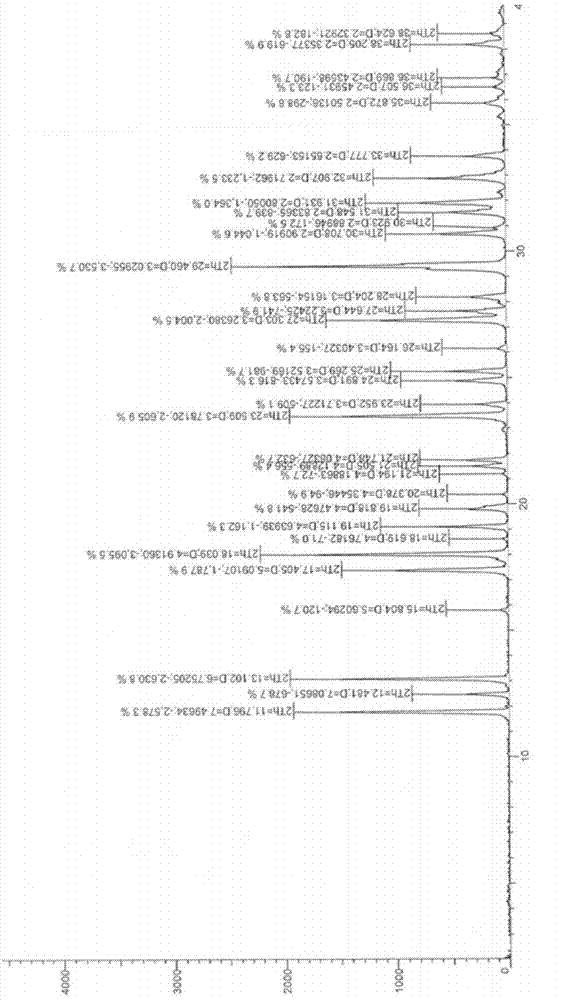
[0025] Instrument model: D8Advance Germany Bruker X-ray powder diffractometer;
[0026] Rays: monochromatic Cu-Kα rays
[0027] Scanning mode: θ / 2θ;
[0028] Scanning range: 2-40°;
[0029] Current: 40mA;
[0030] Voltage: 40kV.
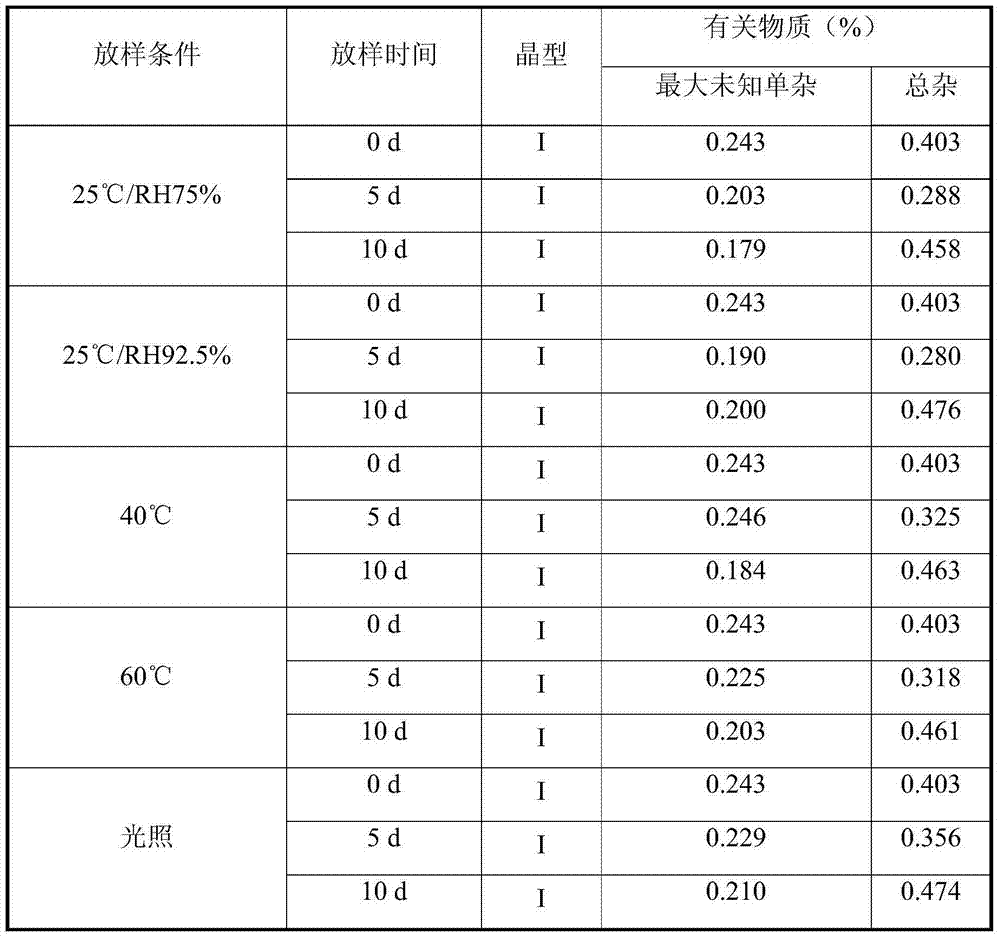
[0031] The comparison of X-ray diffraction data is shown in Table 1.
[0032] Table 1 Statistics of influencing factors of crystal form I of the present invention
[0033]
[0034] It can be seen from the above experiments that the stability of the crystal form of the present invention is very good under various influencing factors, indicating that the crystal form is suitable for the development and application of new drugs.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com