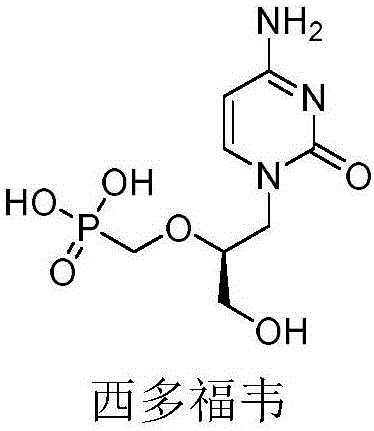
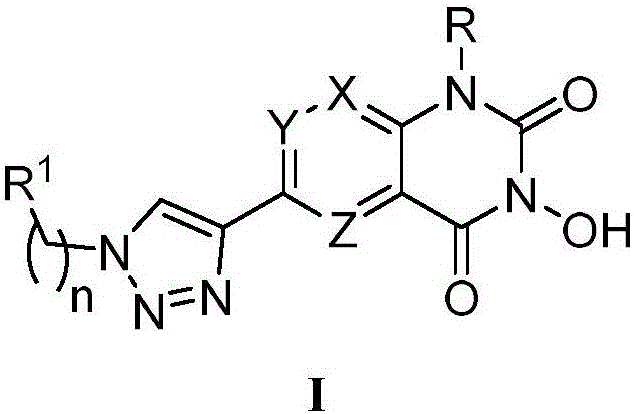
N-hydroxyl heterocycle pyrimindine diketone derivative and preparation method and application thereof
A technology of pyrimidine diketones and derivatives, which can be used in pharmaceutical formulations, medical preparations containing active ingredients, organic chemistry, etc., and can solve problems such as poor therapeutic effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Example 1. Synthesis of key intermediate 3-benzyloxy-6-ethynyl-1-alkylquinazoline-2,4(1H,3H)-dione (5a, 5b)
[0055] The starting material 2-amino-5-iodobenzoic acid (1) (15mmol, 4.0g) was added to 15mL of anhydrous methanol solution, and then 3M concentrated sulfuric acid with a mass fraction of 70% was added to the solution. Heated to reflux for 6h, then stirred at room temperature for 12h. After the reaction was completed, the solvent was distilled off under reduced pressure, and then 100 mL of saturated sodium bicarbonate solution was added to the residue in the bottle, and extracted with ethyl acetate (3×30 mL). The organic layer was separated, washed with saturated brine (3×20 mL), and the organic phase was separated and dried over anhydrous sodium sulfate. After filtration, the solvent was evaporated to dryness under reduced pressure to obtain 3.96 g of crude product of methyl 2-amino-5-iodobenzoate (2), yield: 94.5%, mp: 70-73°C. 1 HNMR (400MHz, DMSO-d6, ppm) ...
Embodiment 2
[0063] Embodiment 2. the synthesis of target product M01-M13 and E01-E13
[0064] The key intermediate 3-benzyloxy-6-ethynyl-1-alkylquinazoline-2,4(1H,3H)-dione (5a, 5b) (0.65mmol) and prepared by substituted benzyl halide Azide (0.65 mmol) was added to a mixed solvent of tert-butanol and water (v / v=1:1, 15 mL). Then, 1M freshly prepared aqueous solution of sodium ascorbate (0.195mmol, 0.04g) and 7.5% aqueous solution of copper sulfate pentahydrate (0.065mmol, 0.017g) were added to this solution. The mixed solution was heated to 65°C and stirred vigorously for 4-12h (TLC detection). After the reaction was completed, a large amount of precipitates were formed, the filter cake was filtered, washed with water, and then recrystallized in methanol to obtain intermediates 6a01-6a13, 6b01-6b13 of the target product.
[0065] Then for different target products, the present invention adopts different synthetic methods. The target products M01-M12 and E01-E12 used method one, while t...
Embodiment 3
[0120] Example 3. In vitro anti-pox virus and anti-adenovirus virus activity test (HEL cell) experiment of the target compound
[0121] Test Principle
[0122] Viruses can only replicate and proliferate in susceptible live animals, chicken embryos or cells. Therefore, animals, chicken embryos or cells can be used to carry out virus culture and drug antiviral tests. Drugs can be judged by observing changes in the pH of cell culture fluid, virus cytopathic effect (CPE), virus titer (PFU), changes in viral genome mRNA levels, changes in chicken embryo allantoic fluid or animal serum, etc. Antiviral test effect.
[0123] Virus CCID 50 Titration principle: After most viruses infect cells cultured in vitro, they often cause changes in cell morphology, such as cell shrinkage, lysis, and cell swelling. This change is called cytopathic effect (CPE), which can be directly measured by Microscopic observation can also be identified and judged by staining. The degree of CPE can indire...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com