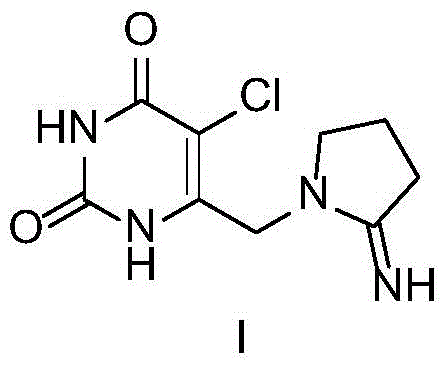
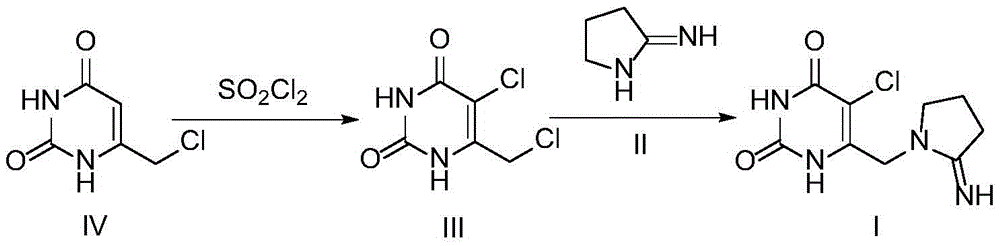
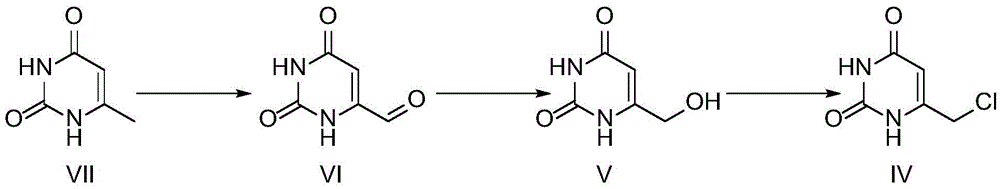
Preparation method of tipiracil intermediate
A technology of pyrimidinedione and chloromethyl is applied in the field of preparation of tippyrimidine intermediates, which can solve the problems of high synthesis and preparation cost, complicated post-processing process, inconvenient product purification and the like, and achieves simplified operation, stable process and production cost. reduced effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] At room temperature, 1000 mL of glacial acetic acid and 150 g of selenium dioxide were successively added into a three-neck flask, and 125 g of compound VII was added into the three-necked flask under stirring, and then the temperature was controlled to 115° C. for reflux reaction for 5 h. After the reaction is completed, filter with suction, add 400 mL of purified water to the filter cake, reflux at 95 ° C for 1 hour, concentrate to dryness after suction filtration, add 400 mL of purified water to it again, make slurry for 1 hour at room temperature, and then filter with suction. After washing with water and absolute ethanol, 90 g of a light yellow solid (compound VI) was obtained after air-drying, with a yield of 68%.
[0030] Step 2:
[0031]
Embodiment 2
[0033] At room temperature, 80 g of compound VI and 500 mL of glacial acetic acid were successively added into a three-necked flask, and 358 g of potassium iodide / potassium iodate (molar ratio 5:1) was slowly added, and the reaction was stirred gently for 4 hours. After the reaction was completed, it was suction-filtered, and the filter cake was washed with purified water and diethyl ether for at least 3 times in sequence, and after air-drying, 132 g of a yellow solid, namely compound VI', was obtained with a yield of 87%.
[0034] Step 3:
[0035]
Embodiment 3
[0037] At room temperature, add 1000mL of ethanol and 100g of compound VI' into the three-necked flask successively, control the temperature in the range of 5-10°C with a low-temperature water bath, add 50g of sodium borohydride into the three-necked flask at a uniform speed, and slowly raise the temperature to room temperature for 1h Then continue to react for 2h. After the reaction was completed, hydrochloric acid was added dropwise to the reaction mixture, the pH was adjusted to about 2, and the solvent was distilled off under reduced pressure. After the solvent was removed, an appropriate amount of purified water was added to stir for beating for 1 hour, and then suction filtered. The filter cake was washed with purified water and ether in turn, and after blast drying, 84 g of a yellow-white solid was obtained, namely compound V', with a yield of 84%.
[0038] Step 4:
[0039]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com