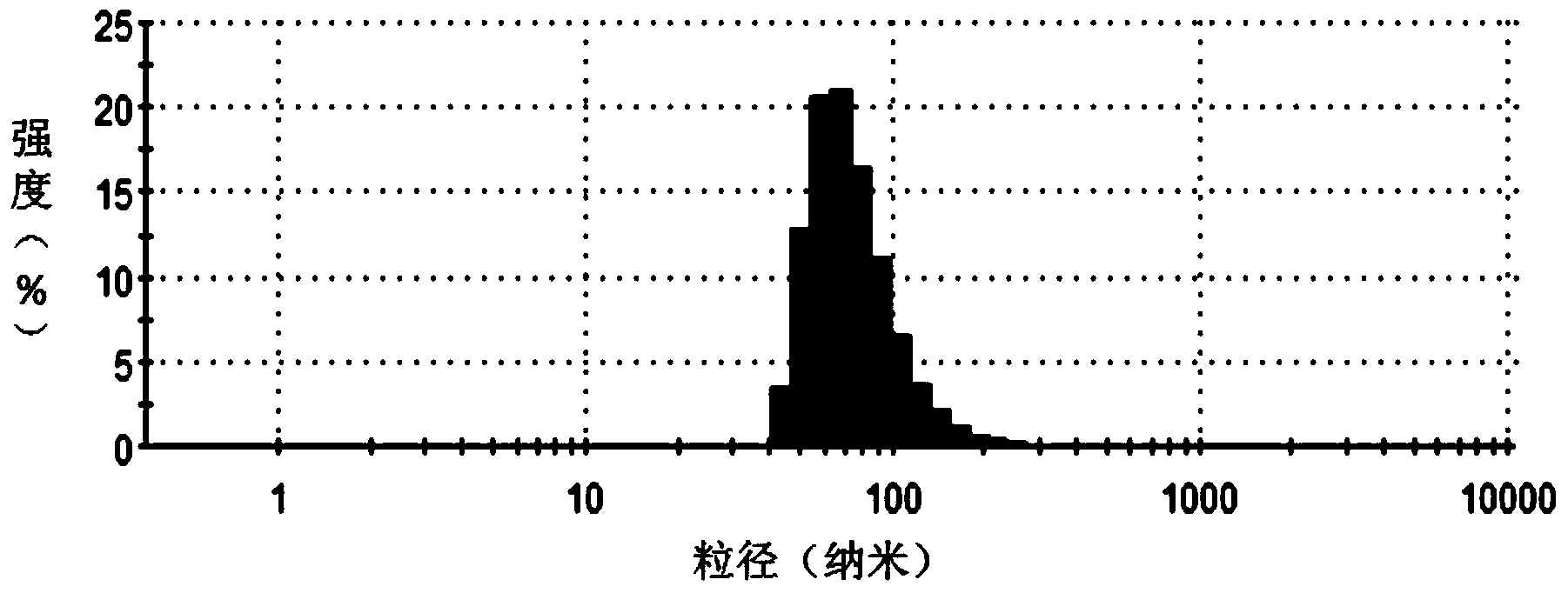
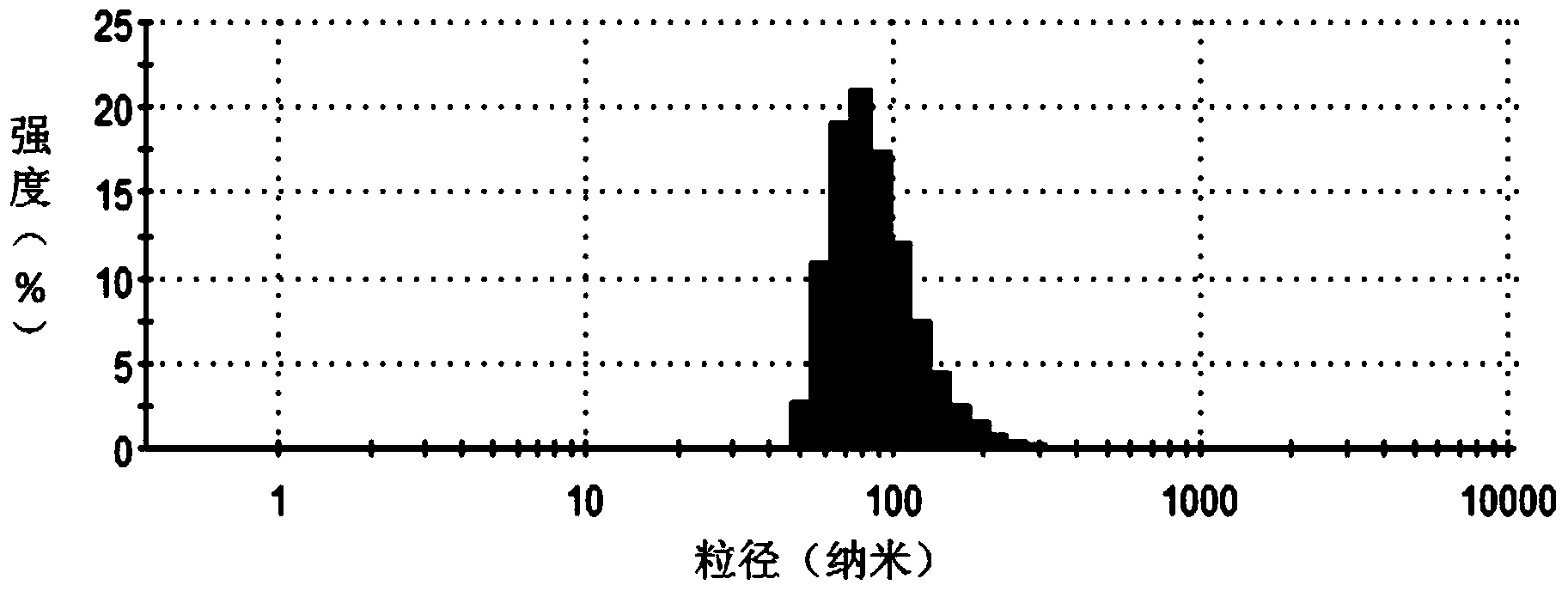
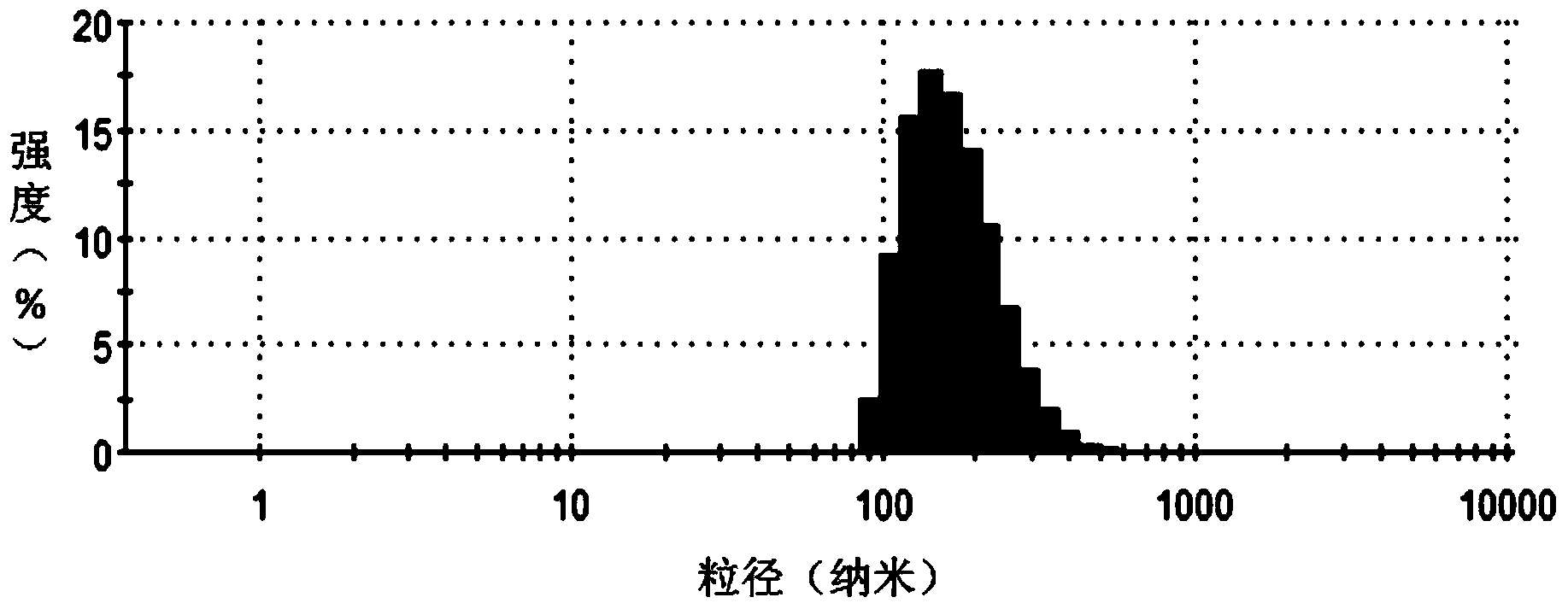
Method for encapsulating hydrophobic antitumor drug by amphiphilic medicament and preparation
An anti-tumor drug and amphiphilic technology, applied in the field of anti-tumor drug preparation, can solve the problems of poor solubility, poor water solubility, and low drug entrapment rate, and achieve the effects of rapid release, simple steps, and overcoming low drug load.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] (1) Dissolve irinotecan hydrochloride (10 mg) and SN-38 (5 mg) in organic solvent dimethyl sulfoxide (80 microliters), the amount of organic solvent added is enough to completely dissolve the drug at room temperature, and mix well.
[0049] (2) Add a certain amount of water (10.8mL) to the solution obtained in step (1), and sonicate for 5min at the same time. The equivalent dose of SN-38 in the solution (SN38equivalent) is 1mg / mL.
[0050] (3) Put the solution obtained in step (2) in a dialysis membrane (molecular weight cut-off 1000), put it in a phosphate buffer solution or pure water with pH=7.0-7.4, and quickly dialyze it for 6 hours at room temperature to remove the organic solvent. Nanoemulsion of SN-38 preparation (drug loading efficiency is basically 100%).
[0051] (4) 6%wt D-trehalose was added to the obtained SN-38 emulsion as a lyoprotectant, and then freeze-dried to prepare irinotecan hydrochloride-loaded nano drug injection powder of SN-38.
[0052] The ...
Embodiment 2
[0055] The irinotecan hydrochloride in embodiment 1 is replaced by topotecan hydrochloride or doxorubicin hydrochloride, the added molar weight is the same as irinotecan hydrochloride, and other operations are the same as in embodiment 1, and topotecan hydrochloride or doxorubicin hydrochloride are prepared. Nano drug injection powder or injection emulsion assembled by mycin and SN-38.
[0056] Correspondingly, nanomedicines with different drug loadings can be prepared according to Example 1 and Example 2 as required.
Embodiment 3
[0058] According to the molar ratio of Example 1, topotecan hydrochloride was used to replace irinotecan hydrochloride, paclitaxel was used to replace SN-38, and other operating steps were the same as in Example 1 to prepare topotecan hydrochloride and paclitaxel assembled nanomedicine injection powder or injection emulsion.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Average size | aaaaa | aaaaa |
| Average size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com