Dechlorination method of zinc sulfate solution
A technology of zinc sulfate solution and acid solution, applied in the direction of improving process efficiency, can solve the problems of difficult processing of back-extraction liquid, high production cost and high degree of dechlorination, so as to enhance market competitiveness, broaden the source of raw materials, and simplify operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
no. 1 example
[0030] Present embodiment is the first example of the dechlorination method of a kind of zinc sulfate solution of the present invention, comprises the steps:
[0031] (1) Acid preparation: 90 cubic meters of zinc sulfate solution containing 100 g / L of zinc and 0.5 g / L of chlorine, mixed with 1 ton of sulfuric acid to obtain 90.5 cubic meters of acidic solution with a sulfuric acid concentration of 20 g / L;
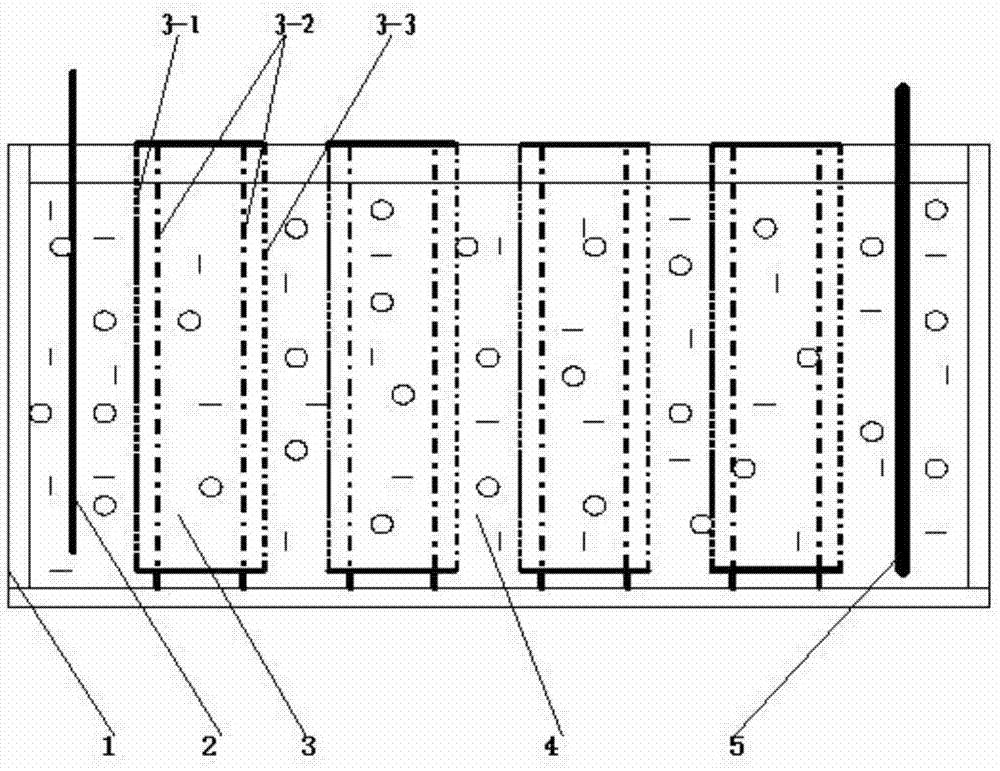
[0032] (2) Electrolysis: In a four-diaphragm electrolytic cell equipped with 15 diaphragm cells, the acidic solution is heated at a temperature of 25°C and a current density of 50A / m 2 , The total tank voltage is 12V, and the effective area of each diaphragm is 680mm*670mm=0.6m 2 , Liquid inlet speed 0.4m 3 Electrolysis is carried out under the condition of / h, and the output is 0.37m 3 / h of chlorine containing 0.1 g / l solution and get 0.03m 3 The solution containing 5.4 g / L of chlorine per hour, and the solution containing 0.1 g / L of chlorine are used to produce elec...
no. 2 example
[0036] Present embodiment is the second example of the dechlorination method of a kind of zinc sulfate solution of the present invention, comprises the steps:
[0037] (1) Acid preparation: 120 cubic meters of zinc sulfate solution containing 130 g / l of zinc and 3 g / l of chlorine, mixed with 3 tons of sulfuric acid to obtain 121 m3 of acidic solution with a sulfuric acid concentration of 45 g / l;
[0038] (2) Electrolysis: In a four-diaphragm electrolytic cell equipped with 20 diaphragm cells, the acid solution is heated at a temperature of 30°C and a current density of 120A / m 2 , The total tank voltage is 25V, and the effective area of each diaphragm is 980mm*970mm=0.95m 2 , Liquid inlet speed 0.4m 3 Electrolyzed under the condition of / h, the output is 0.32m 3 / h of chlorine containing 0.25 g / L low chlorine solution and 0.08m 3 Chlorine-containing 15 g / liter chlorine solution per hour, chlorine-containing 0.25 g / liter solution is used to produce electric zinc;
[0039](...
no. 3 example
[0042] Present embodiment is the third example of the dechlorination method of a kind of zinc sulfate solution of the present invention, comprises the steps:
[0043] (1) Acid preparation: 100 cubic meters of zinc sulfate solution containing 150 g / L of zinc and 5 g / L of chlorine, mixed with 7.7 tons of sulfuric acid to obtain 146 g / L of zinc, 75 g / L of sulfuric acid, and 103 cubic meters of acidic solution of 4.85 grams per liter of chlorine;
[0044] (2) Electrolysis: In a four-diaphragm electrolytic cell equipped with 20 diaphragm cells, the acidic solution is heated at a temperature of 35°C and a current density of 200A / m 2 , The total tank voltage is 24V, and the effective area of each diaphragm is 980mm*970mm=0.95m 2 , Liquid inlet speed 0.35m 3 Electrolysis is carried out under the condition of / h, and the output is 0.27m 3 / h of chlorine containing 0.3 g / l solution and 0.08m 3 The solution containing 20 g / L of chlorine per hour, and the solution containing 0.3 g / L...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com