Novel pharmaceutical protein DRACO and applications thereof in prevention and treatment of porcine reproductive and respiratory syndrome
A drug and protein technology, applied in the field of molecular biology, can solve the problems of weak cellular immunity, dispersing toxins, and short immune period, and achieve good antiviral effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1 DRACO Gene Design Synthesis and Expression Analysis
[0047] 1. Design and synthesis of DRACO gene: According to NCBI (http: / / www.ncbi.nlm.nih.gov / ), the partial sequence of porcine protein kinase R (Protein Kinase R, PKR) (sequence number is AB104654.1) gene (as shown in SEQ ID NO.4) and the gene partial sequence of apoptosis protease activating factor 1 (apoptotic protease activating factor 1, Apaf-1, sequence number is AF013263.1) (as shown in SEQ ID NO.5) , N-terminal plus a transducing peptide sequence PTD, the amino acid sequence of PTD is shown in SEQ ID NO.3, and the DRACO protein coding gene is designed, and the sequence is shown in SEQ ID NO.1. The amino acid sequence of the DRACO protein encoded by the gene is SEQ ID NO.2. The designed DRACO sequence was sent to Sangon Bioengineering (Shanghai) Co., Ltd. (http: / / sangon.com / ) for synthesis.
[0048] 2. Construction of DRACO gene recombinant prokaryotic expression strain: the DRACO gene synthesize...
Embodiment 2
[0052] Example 2 Purification of DRACO protein
[0053] The DRACO protein was purified by His-tag through the column, and the BCA protein quantification kit (purchased from Thermo Scientific, catalog number: 23227) was used to determine the concentration of the DRACO protein.
[0054] 1. Prepare the protein sample, phosphate buffer, Binding buffer, Washing buffer, Elution buffer, 20% ethanol, distilled water, and install the Ni-NTA chromatography column on the iron stand.
[0055] 2. Use a syringe to add ddH2O 4 times the column volume (5mL) to wash the Ni-NTA chromatography column, equilibrate the chromatography column, and use a Binding buffer 4 times the volume of the column to balance the column. The Binding buffer plays an adhesion role, the purpose is For the next step, the filtered His-tagged protein adhered to Ni-NTA resin, and the filtered His-tagged protein was added to the chromatographic column equipped with Ni-NTA resin in the same way.
[0056] 3. Use 4 times ...
Embodiment 3
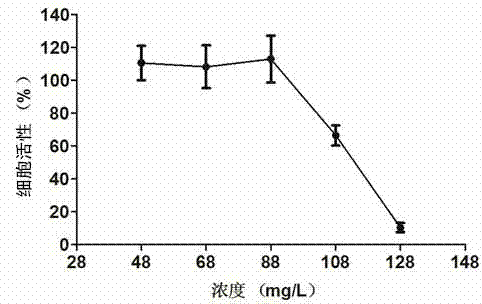
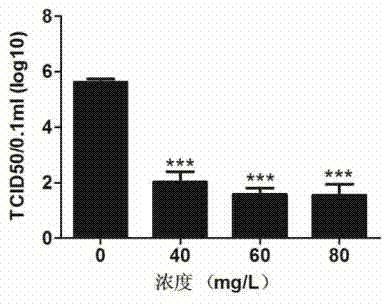
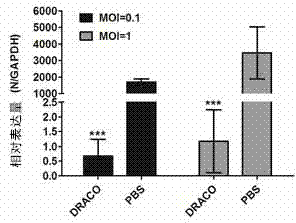
[0058] Example 3 DRACO Cytotoxicity Test
[0059]AlamarBlue (purchased from Invitrogen) is used as an indicator of living cell metabolism. Under the mitochondrial enzymatic reduction reaction, a measurable fluorescent metabolite will be produced, and the cell activity can be monitored by measuring its fluorescence intensity. Cultivate Marc-145 cells with DMEM medium containing 10% fetal bovine serum to 60-70%, discard the medium, add nutrient solution containing DRACO times dilution for 36 hours, set the PBS control group, and then add 10% ( V / V) ratio AlamarBlue continued to incubate for 3 hours, and read the fluorescence values of excitation light at 540nm and emission light at 590nm with a multi-functional microplate reader to make a DRACO cytotoxicity map. See attached image 3 , taking the cell viability of the PBS control group as 100%, the fluorescence value of the cells treated with DRACO in multiple dilutions compared with the fluorescence value of the PBS contro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com