Method for preparing fluorinated alkene by performing dehydrochlorination on chlorofluorocarbon under action of catalyst
A technology for dehydrochlorination and fluorine-containing olefins, which is applied in the direction of dehydrohalogenation preparation, chemical instruments and methods, physical/chemical process catalysts, etc., can solve the problems of short service life of catalysts, high production costs, low yields, etc., and achieve reduction Operating cost, low specification requirement, effect of increased selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1: adopt impregnation method to prepare catalyst
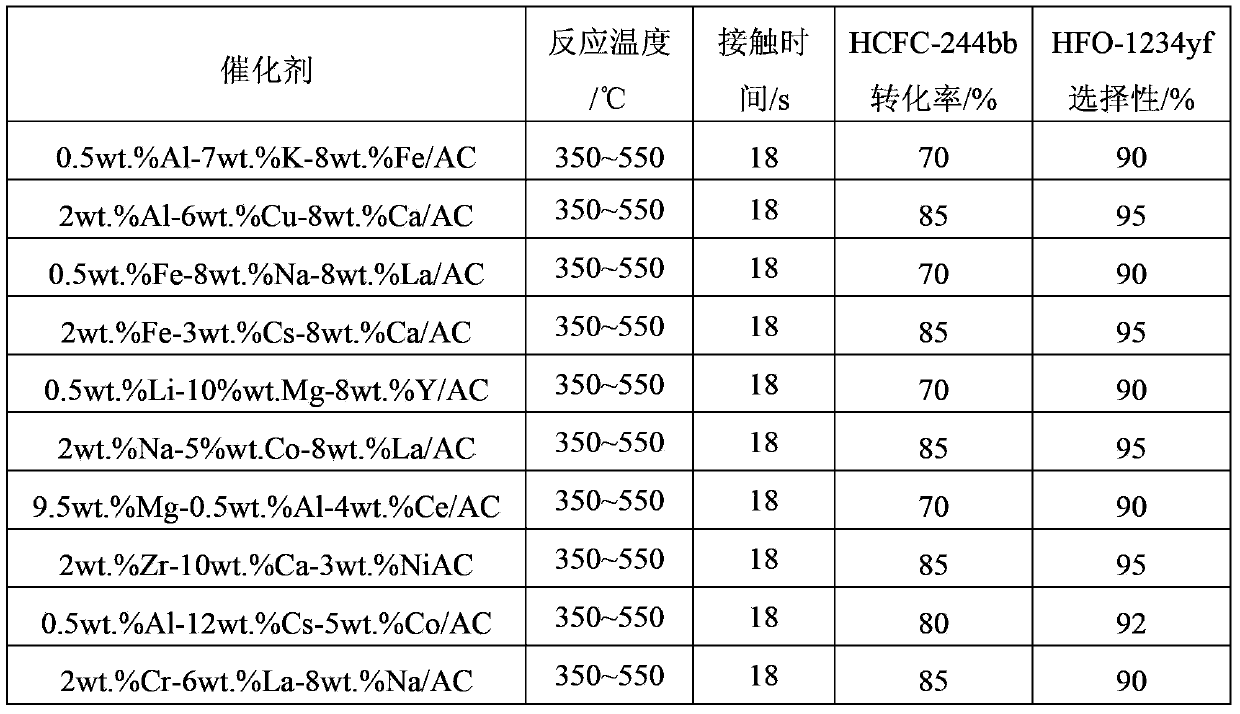
[0044] (1) 11.0g magnesium nitrate (Mg(NO 3 ) 2 ), 24.0g ytterbium nitrate (Y 2 (NO 3 ) 3 ), 10.0g ferric chloride was dissolved in 80ml distilled water to make a solution, and then the specific surface area was 860m 2 / g of coal-based activated carbon is added to the above solution as a carrier, after immersion for 8 hours, it is dried at 120°C for more than 12 hours, and then the precursor is roasted in a muffle furnace at 400°C for 6 hours to obtain a catalyst. The mass percentage is : 5%wt.Mg-1.5%wt.Y-3.0%wt.Fe / AC, spare.
[0045] (2) The preparation process of the catalyst is basically the same as (1), except that the magnesium nitrate is changed to cesium chloride, the ytterbium nitrate is changed to aluminum sulfate, the ferric chloride is changed to lanthanum nitrate, the immersion time is changed to 6h, and the drying temperature is Change it to 80°C, change the calcination temperature to 450°C...
Embodiment 2
[0067] A series of catalysts prepared by the examples are used as dehydrochlorination catalysts, and 60ml of catalysts are loaded into a fixed-bed tubular reactor with a diameter of Φ38mm. At a reaction temperature of 350°C to 850°C, 2 -Mixture of chloro-1,1,1,2-tetrafluoropropane, 15wt.% of 2-chloro-3,3,3-trifluoropropene and 5wt.% of HF, through the catalyst bed, the residence time is 18s . The product is washed with water and alkali to remove HCl and HF, and then analyzed by gas chromatography. The analysis method is the area normalization method. Before the reaction, the catalyst was kept at 400°C for 2 hours in a hydrogen atmosphere, then adjusted to the reaction temperature, and the reaction raw materials were switched, and the reaction was carried out for 10 hours. The reaction results are shown in Table 1.
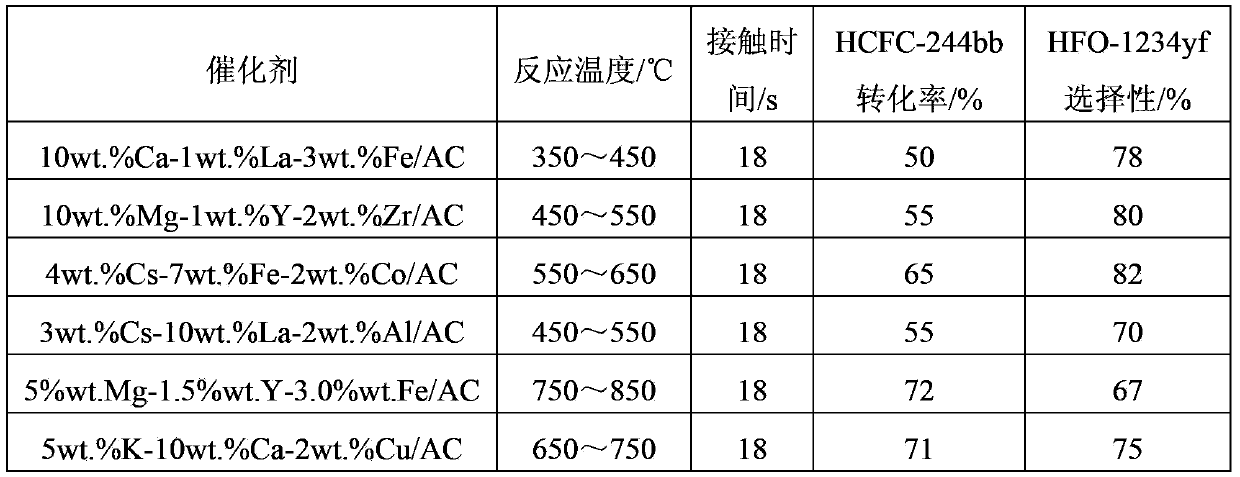
[0068] The reaction result of table 1 embodiment 2
[0069]
[0070] It can be seen from Table 1 that increasing the reaction temperature can effectively incr...
Embodiment 3
[0072] This example uses the same operating conditions as in Example 2, except that the composition of the reaction raw materials is changed to 85wt.% of 2-chloro-1,1,1,2-tetrafluoropropane, 10wt.% of 2-chloro - The mixture of 3,3,3-trifluoropropene and 5wt.% HF, the contact time was changed to 0.5s, 18s, 33s and 60s, the reaction results are shown in Table 2.
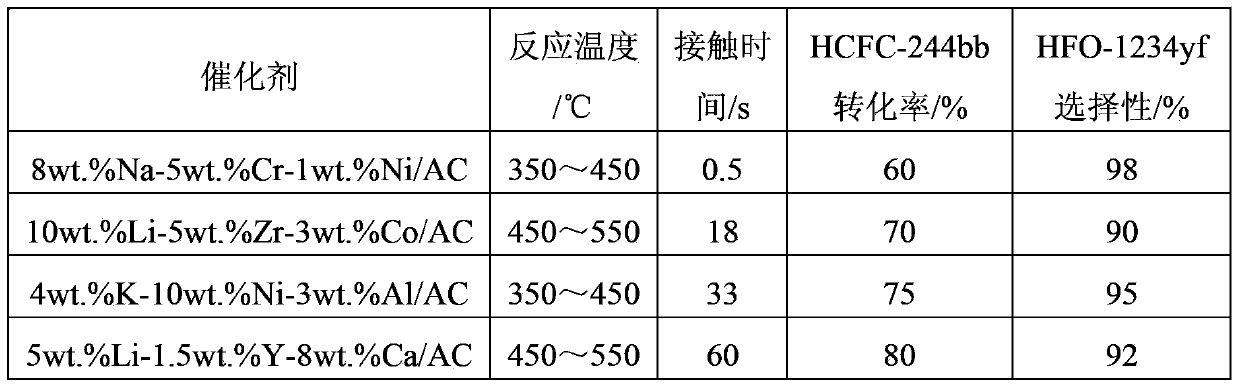
[0073] The reaction result of table 2 embodiment 3
[0074]
[0075] It can be seen from Table 2 that prolonging the reaction contact time can significantly increase the conversion of raw materials, but will partially reduce the selectivity of the target product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com