High-temperature alkaline xylanase XYN11A as well as gene and application thereof
A technology of xylanase and mannanase, applied in the field of genetic engineering, can solve problems such as loss, low expression limit industrial production prospects, and inability to adapt to alkaline environment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Cloning of Example 1 Xylanase Encoding Gene Xyn11A
[0050] The xylanase gene xyn11A is derived from Humicola. Extraction of Humicola Genomic DNA:
[0051] After 3 days of cultivation, the mycelium was centrifuged at high speed and taken into a mortar, frozen in liquid nitrogen and ground for 5 minutes, then the grinding solution was placed in a 50mL centrifuge tube, 2mL of CTAB extract was added, and lysed in a water bath at 70°C for 2h, every 10min Mix once and centrifuge at 12000rpm for 10min at 4°C. The supernatant was extracted in phenol / chloroform to remove impurity proteins, and then an equal volume of isopropanol was added to the supernatant. After standing at room temperature for 10 minutes, centrifuge at 12,000 rpm for 10 minutes at 4°C. The supernatant was discarded, the precipitate was washed twice with 70% ethanol, dried in vacuo, dissolved in 0.2mL TE, and stored at -20°C for later use.
[0052] Since the whole gene sequence of the Humicola sp.S8 strain...
Embodiment 2
[0057] The preparation of embodiment 2 recombinant xylanase
[0058] The expression vector pPIC9 was subjected to double enzyme digestion (EcoR I+Not I), and at the same time, the amplified xyn11A gene encoding xylanase (without signal peptide) was subjected to double enzyme digestion (EcoR I+Not I), and the xyn11A gene encoding the xylanase was cut out. The polycanase gene fragment is connected with the expression vector pPIC9 to obtain the recombinant plasmid pPIC9-xyn11A containing the xyn11A gene xyn11A and transform the Pichia pastoris GS115 to obtain the recombinant Pichia pastoris strain GS115 / xyn11A.
[0059] The GS115 strain containing the recombinant plasmid was inoculated in 400mL of BMGY culture medium, shaken at 250rpm at 30°C for 48h, and then collected by centrifugation. Then resuspend in 200mL BMMY medium, shake culture at 250rpm at 30°C. After 72 hours of induction, the supernatant was collected by centrifugation, and the activity of xylanase was determined. ...
Embodiment 3
[0061] The activity analysis of embodiment 3 recombinant xylanase
[0062] The recombinant xylanase produced by the recombinant strain GS115 / XYN11A was purified, and the activity was analyzed by DNS method.
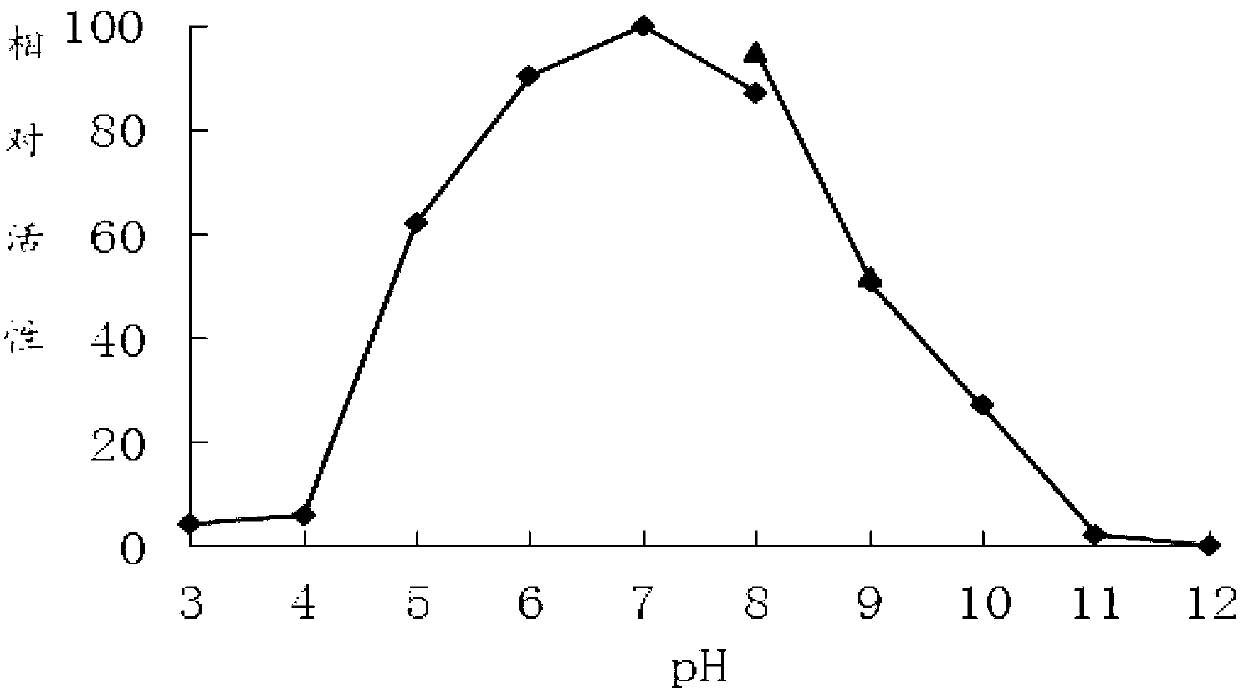
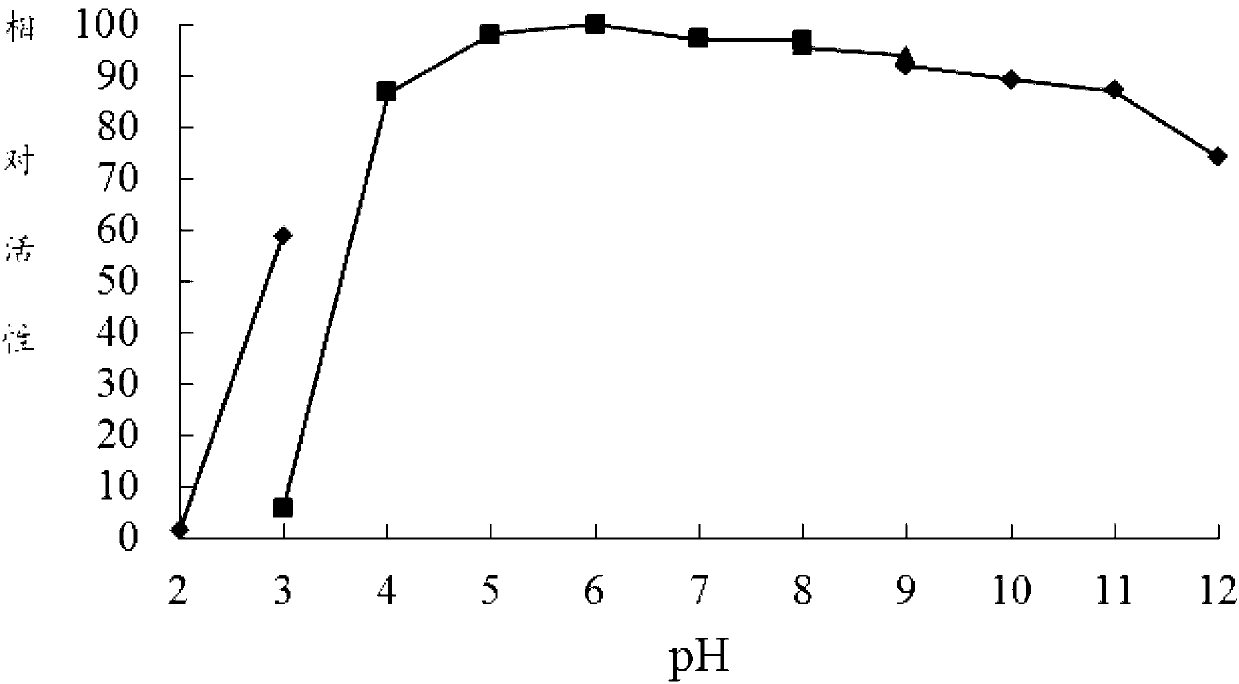
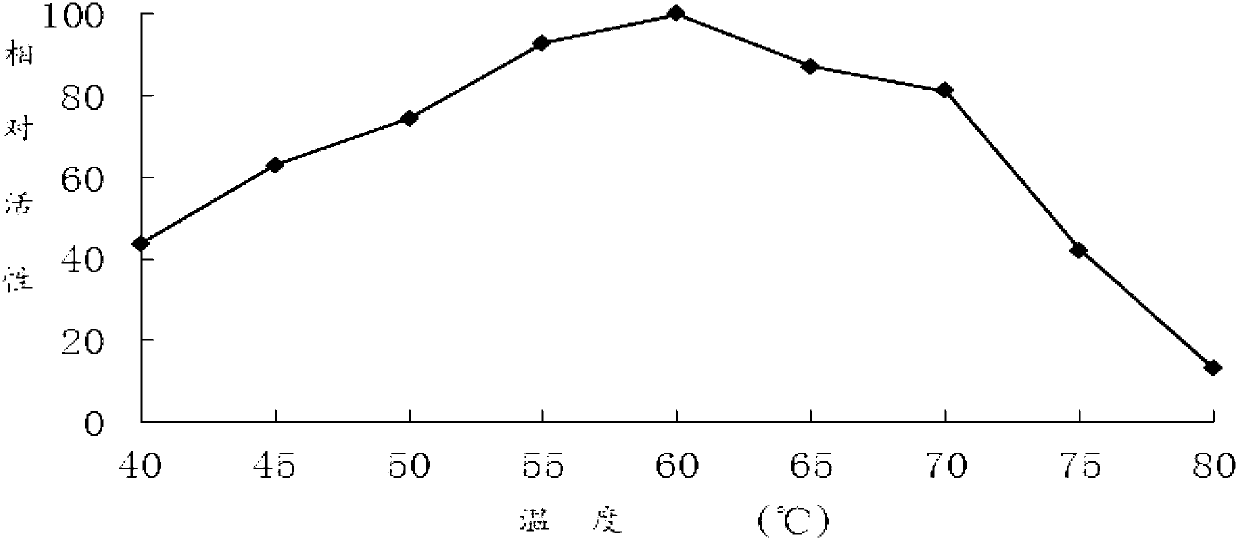
[0063] DNS method: The specific method is as follows: at pH 7.0 and 60°C, 1 mL of reaction system includes 100 μL of appropriate diluted enzyme solution, 900 μL of substrate, reacted for 10 minutes, added 1.5 mL of DNS to terminate the reaction, and boiled for 5 minutes. After cooling, the OD value was measured at 540 nm. One enzyme activity unit (U) is defined as the amount of enzyme that releases 1 μmol of reducing sugar per minute under given conditions.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com