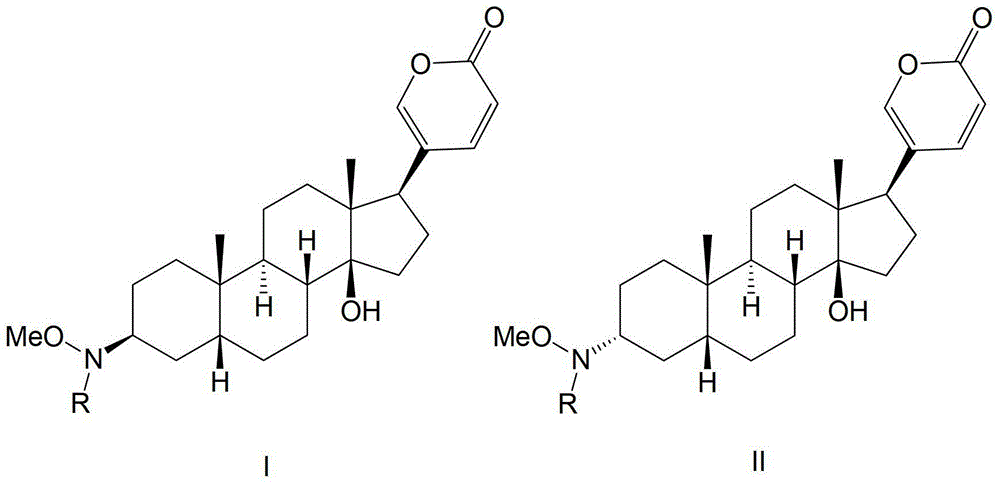
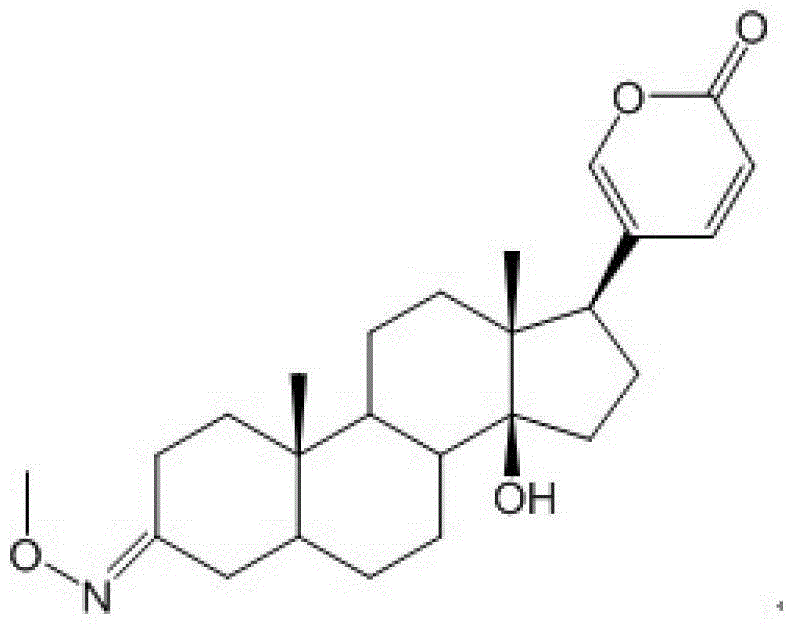
Glycosylated derivatives of bufolin and its preparation method and application in the preparation of antitumor drugs
An anti-tumor drug, bufalin technology, applied in the direction of anti-tumor drugs, drug combinations, steroids, etc., can solve the problems of poor selectivity, poor water solubility, etc., and achieve low toxic and side effects, reduce toxic and side effects, and high yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The synthesis of bufolin-3-N-D-glucoside comprises the following steps:
[0036] (1) Put bufalin (4.13g, 0.0107mol) in a 50ml round bottom flask, add CH 2 Cl 2 (35ml) was stirred until completely dissolved, slowly added PCC (pyridinium chlorochromate hydrochloride) (4.60g, 0.0214mol), and stirred at a constant speed at room temperature for 2.5h. The mixture was filtered, and the filtrate was concentrated under reduced pressure. The reaction product Bufalinone (3.9 g, 0.0102 mol, yield 95%) was obtained by silica gel column chromatography (cyclohexane / ethyl acetate mixture at a volume ratio of 1:1).
[0037] Structural analysis of the product obtained in step (1).
[0038] Physical and chemical properties: white powder (TLC R f =0.35 cyclohexane / ethyl acetate 1:1).
[0039] Spectral information: ESI-MS m / z: 385.5[M+H] + , 407.4[M+Na] + , 791.4[2M+H] + , 823.3[2M+Na] + . 1 H NMR (CDCl 3 , 300MHz) δ7.83 (1H, dd, J=9.7, 2.6Hz, H-22), 7.22 (1H, d, J=2.6Hz, H-21), ...
Embodiment 2
[0094] The synthesis of bufolin-L-glucoside comprises the following steps:
[0095] (1)-(3) The method for preparing aglycon is the same as that in Example 1.
[0096] (4) Add aglycon III (46mg, 0.108mmol) and L-glucose (39.0mg, 0.217mmol) into a reaction tube, dissolve in DMF / AcOH3:1 (1204μl), and stir at a constant speed at 40°C for 48h. After the reaction, the mixture was concentrated and dried under reduced pressure to remove the reaction solvent, and the sample was dissolved in methanol, and the sample was separated and purified by a preparative high-performance liquid chromatography column (waters, C18, 5 μm, 20×250 mm), with a flow rate of 8 ml / min and a detection wavelength of 296 nm. Use 45% acetonitrile / water solution as mobile phase, retention time t R =16.02min, the glycosylated product glycoside 2a (26mg, 0.045mmol, yield 42%) was obtained.
[0097] The structure of the glycosylation product 2a obtained in (4) was identified.
[0098] Physical and chemical prop...
Embodiment 3
[0114] The synthesis of bufolin-D-fucoside comprises the following steps:
[0115] (1)-(3) are the same as the steps in Example 1.
[0116] (4) Add aglycon III (42mg, 0.101mmol) and D-fucose (33.6mg, 0.205mmol) into a reaction tube, dissolve in DMF / AcOH 3:1 (1122μl), and stir at a constant speed at 40°C for 48h. After the reaction, the mixture was concentrated and dried under reduced pressure to remove the reaction solvent, and the sample was dissolved in methanol, and the sample was separated and purified by a preparative high-performance liquid chromatography column (waters, C18, 5 μm, 20×250 mm), with a flow rate of 8 ml / min and a detection wavelength of 296 nm. Use 45% acetonitrile / water solution as mobile phase, retention time t R =11.57min, the glycosylated product glycoside 3a (22mg, 0.039mmol, yield 39%) was obtained.
[0117] The structure of the glycosylation product 3a obtained in (4) was identified.
[0118] Physical and chemical properties: white powder (TLC R ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com