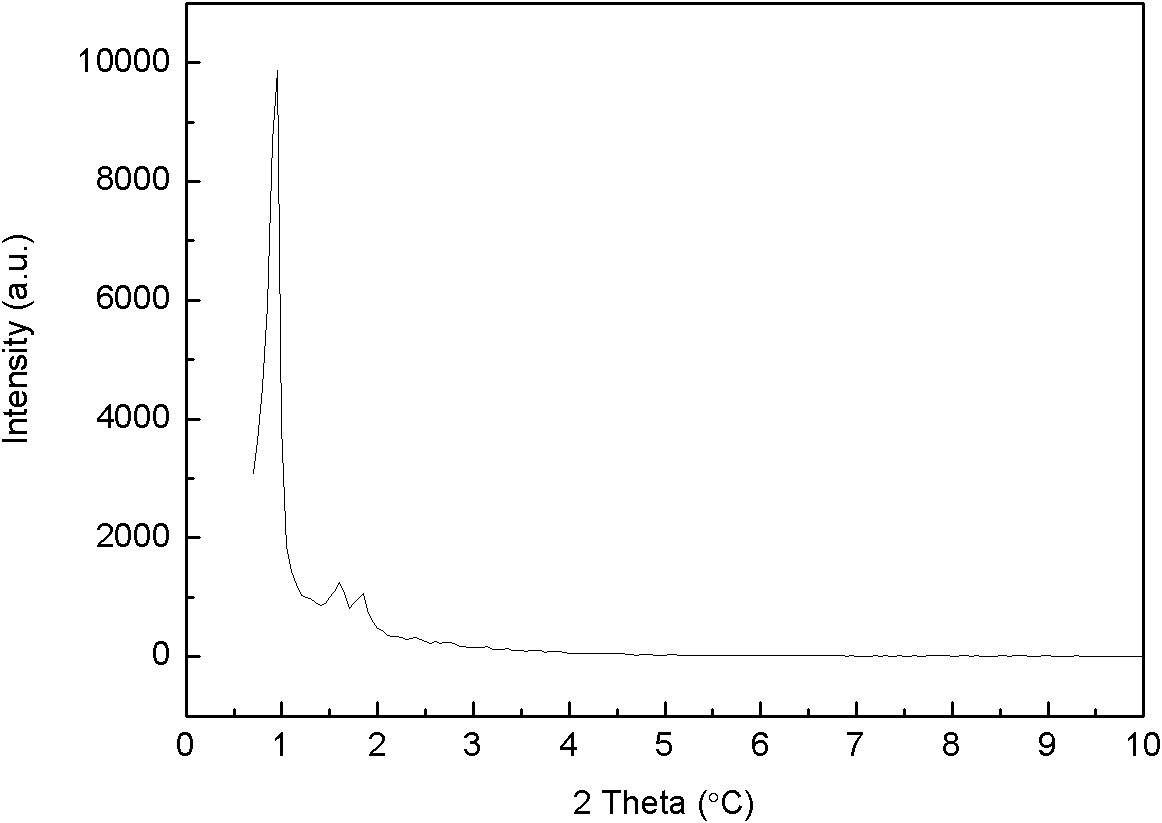
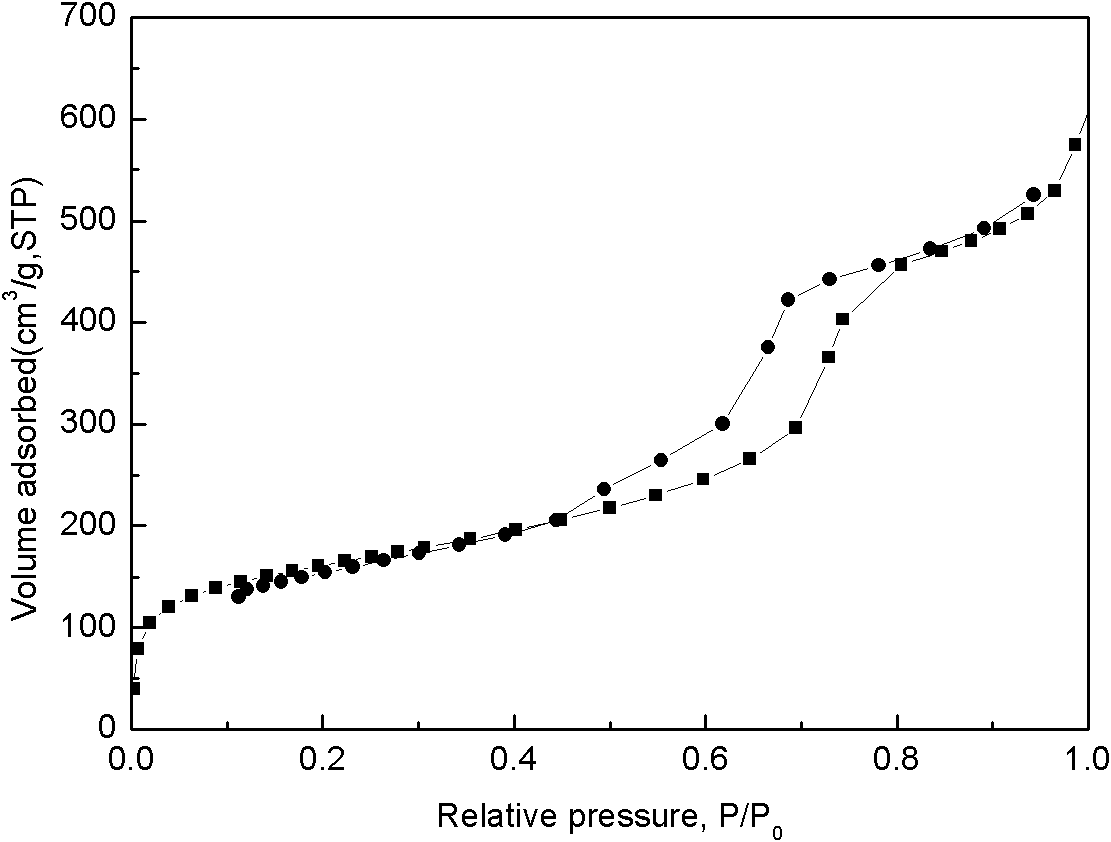
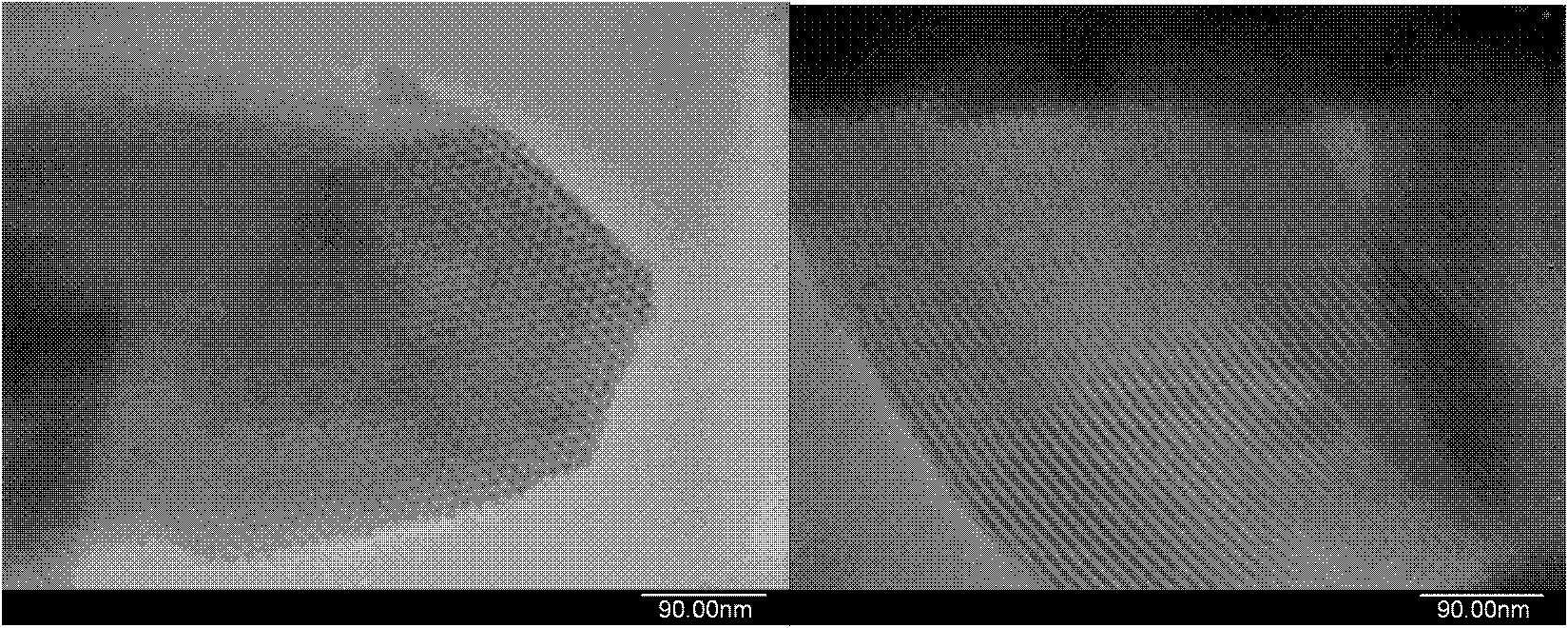
A method for removing cyanide-containing waste gas
A waste gas and removal technology, which is applied in the field of removal of cyanide-containing waste gas and catalytic combustion method to remove cyanide-containing waste gas, can solve the problems of low specific surface area of alumina carrier, poor dispersion of active components, and higher requirements for removal conditions , to achieve the effect of good low temperature activity, improved dispersion, and no by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: take by weighing 0.0756gCu (NO 3 ) 2 ·3H 2 O (chemically pure reagent), and dissolve it in water to form an impregnating solution, then weigh 1g of SBA-15 mesoporous molecular sieve and add it to the impregnating solution, stir in a water bath at 40°C for 24 hours, and then use a rotary evaporator to remove the impregnating solution. Finally, the above-mentioned supported catalyst was placed in an air atmosphere at a rate of 2 °C / min to 550 °C for 10 hours to obtain a Cu / SBA-15 catalyst with a mass fraction of 5% copper. The prepared catalyst was placed in a miniature fixed-bed quartz reactor, and then the reactor was heated to 450°C, and a CH 3 CN(1vol%), O 2 (5vol%) and N 2 (As a balance gas) mixed gas, the air velocity of the mixed gas is 20000h -1, using an American Nicolet Nexus 470 infrared spectrometer with a 2.4m optical path gas analysis cell for online gas quantitative analysis, so as to obtain the conversion rate of acetonitrile and the yie...
Embodiment 2
[0027] Embodiment 2: take by weighing 0.1538gCr (NO 3 ) 3 9H 2 O, and dissolve it in water to make an impregnating liquid, then weigh 1g of SBA-16 mesoporous molecular sieve and add it to the impregnating liquid, stir in a water bath at 40°C for 24 hours, then use a rotary evaporator to remove the water in the impregnating liquid, and finally The above-mentioned supported catalyst was placed in an air atmosphere at a rate of 2 °C / min to 550 °C and calcined for 10 hours to obtain a Cr / SBA-15 catalyst with a mass fraction of 2% chromium supported. Put the prepared catalyst in a miniature fixed-bed quartz reactor, then raise the temperature of the reactor to 500°C, and pass through the reactor containing CH 3 CN(1vol%), O 2 (5vol%) and N 2 (As a balance gas) mixed gas, the air velocity of the mixed gas is 20000h -1 , using an American Nicolet Nexus 470 infrared spectrometer with a 2.4m optical path gas analysis cell for online gas quantitative analysis, so as to obtain the c...
Embodiment 3
[0028] Embodiment 3: take by weighing 0.0986gCo(NO 3 ) 2 ·6H 2 O, and dissolve it in water to form an impregnating liquid, then weigh 1g of KIT-5 mesoporous molecular sieve and add it to the impregnating liquid, stir in a water bath at 40°C for 24 hours, then use a rotary evaporator to remove the water in the impregnating liquid, and finally The above-mentioned supported catalyst was placed in an air atmosphere at a rate of 2 °C / min to 550 °C and calcined for 10 hours to obtain a Co / KIT-5 catalyst with a mass fraction of 2% cobalt. The prepared catalyst was placed in a miniature fixed-bed quartz reactor, and then the reactor was heated to 400°C, and a CH 3 CN(1vol%), O 2 (5vol%) and N 2 (As a balance gas) mixed gas, the air velocity of the mixed gas is 20000h -1 , using an American NicoletNexus 470 infrared spectrometer with a 2.4m optical path gas analysis cell for online gas quantitative analysis, so as to obtain the conversion rate of acetonitrile and the yield of each...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com