High-dose clinorhomboidal adefovir dipivoxil preparation, and preparation method and application thereof
A technology of adefovir dipivoxil and triclinic crystal is applied in the field of high-dose preparations of triclinic adefovir dipivoxil, which can solve the problems that affect the safety of adefovir dipivoxil, the drug is difficult to exert its original crystal form, and the raw material drug Crystal form damage and other problems, to achieve the effect of ensuring the uniformity of tablet weight/grain weight, ensuring production continuity and high safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
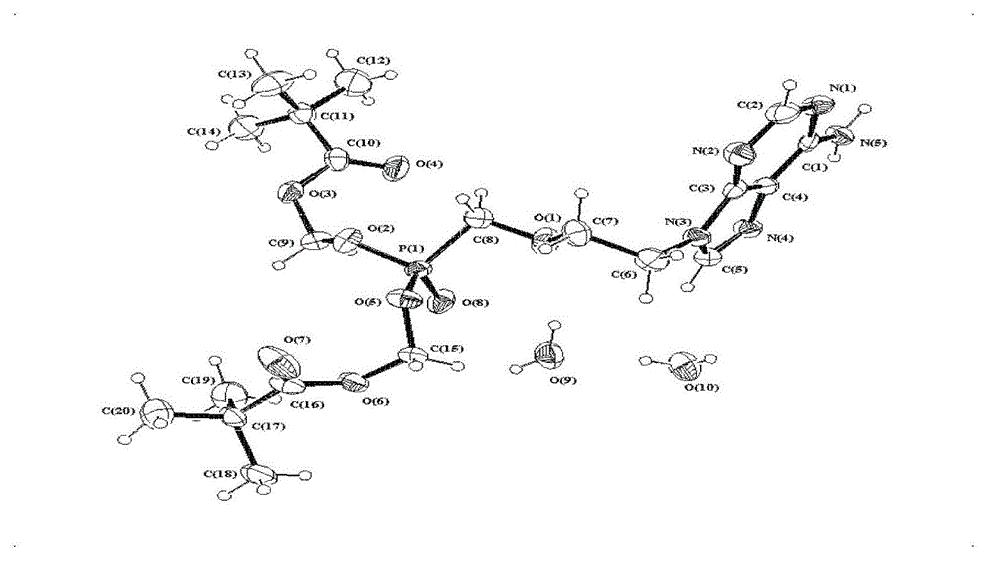
[0048] Embodiment 1: Preparation of specific triclinic adefovir dipivoxil whose space group is P-1
[0049] Drying Reactor N 2 Protection, heating and dissolving 1000g of the crude product of adefovir dipivoxil in 10L of ethanol, filtering off the insolubles while hot, adding diisopropyl ether at 20~25°C until cloudy, then stirring until crystallization is complete, filtering and vacuum drying for 24 hours, Triclinic crystal adefovir dipivoxil was obtained with a yield of 85-90%, a content of ≥99.0% (HPLC), and a melting point of 99-101°C.
[0050] The obtained triclinic adefovir dipivoxil single crystal X-ray diffraction data are as follows: the crystal belongs to the triclinic system, the space group is P-1, and the unit cell parameters are as follows: α=93.798(6)°.β=97.458(6)°.γ=102.897(6)°.Z=2.
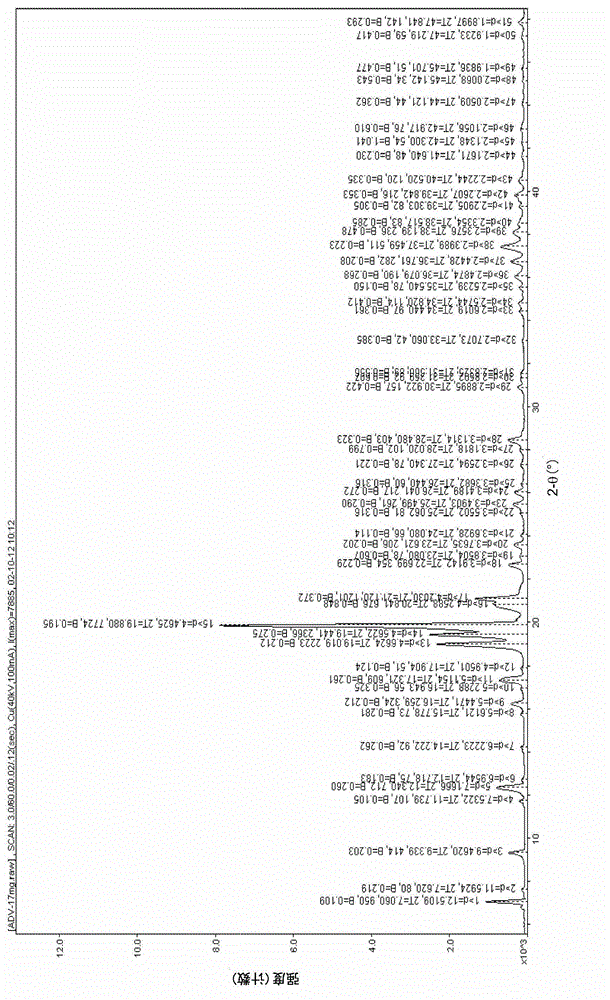
[0051] The data of triclinic adefovir dipivoxil powder X-diffraction are as follows:
[0052]
Embodiment 2
[0053] Example 2: High-dose triclinic adefovir dipivoxil tablet or capsule preparation dry granulation process
[0054] 1. Selection of carriers and excipients:
[0055] Diluent: anhydrous lactose (supplier: Hefei Shanhe Accessories Co., Ltd.; manufacturer: DMV International Co., Ltd.; registration number: F20090027; specification: medicinal (import))
[0056] Adhesive: pregelatinized starch (supplier: Huzhou Zhanwang Pharmaceutical Co., Ltd.; manufacturer: Huzhou Zhanwang Pharmaceutical Co., Ltd.; approval number: Zheyao Zhunzi: F20060057; executive standard: Chinese Pharmacopoeia 2010 edition; specification : medicinal);
[0057] Disintegrant: sodium starch glycolate (supplier: Huzhou Zhanwang Pharmaceutical Co., Ltd.; manufacturer: Huzhou Zhanwang Pharmaceutical Co., Ltd.; approval number: F20060059; executive standard: Chinese Pharmacopoeia 2010 edition; specification: medicinal) ;
[0058] Lubricant: Talc powder (supplier: Shanghai New Pioneer Huakang Pharmaceutical Co...
Embodiment 3
[0078] Embodiment 3: triclinic adefovir dipivoxil tablet or capsule and content detection of related substances:
[0079] Determination according to high performance liquid chromatography (Chinese Pharmacopoeia 2010 edition two appendix VD).
[0080] Chromatographic conditions and system suitability test use mixed anion exchange carbon octaalkyl bonded silica gel as filler (such as Alltech, Mix-modeC8 / Anion, 7μm, 25cm×4.6mm); use 0.2moL / L phosphate buffer ( Take 9.2g of anhydrous dipotassium hydrogen phosphate and 47.2g of anhydrous potassium dihydrogen phosphate, add water to dissolve and dilute to 2000ml, adjust the pH value to 6.0 with phosphoric acid or potassium hydroxide solution)-acetonitrile (70:30) as mobile phase A , using 0.2moL / L phosphate buffer (PH 6.0)-acetonitrile (50:50) as mobile phase B, perform linear gradient elution according to the following procedure, the flow rate is 1.2ml per minute; the detection wavelength is 260nm.
[0081] Determination method Ta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com