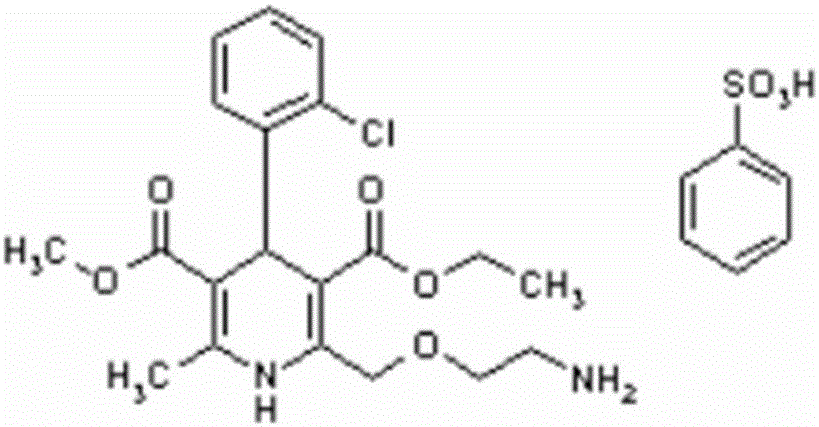
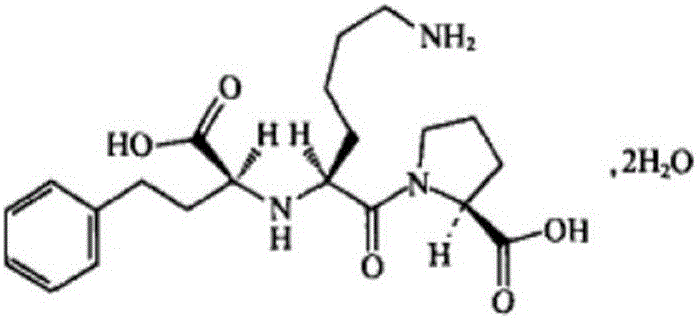
Medicine containing amlodipine besylate and lisinopril dehydrate and preparation method thereof
A technology of amlodipine besylate and dihydrate, which is applied in the field of medicine, can solve the problems of unqualified tablet content uniformity, unqualified friability, complicated production process, etc., and achieve the reduction of types of excipients, high production efficiency, easy-to-handle effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
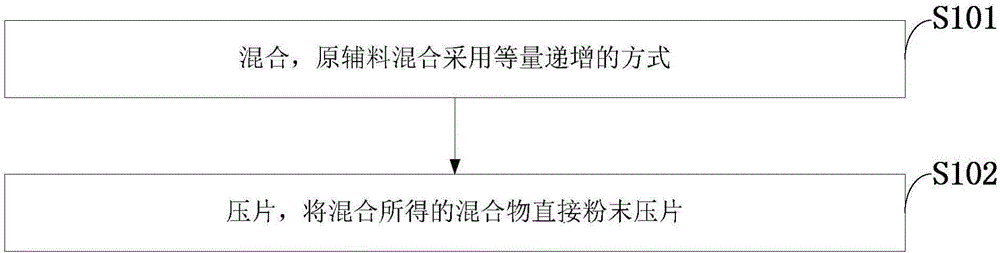
[0034] Such as figure 1 As shown, the preparation method of the medicine containing amlodipine besylate and lisinopril dihydrate of the embodiment of the present invention comprises the following steps:
[0035] S101: Mixing, raw and auxiliary materials are mixed in an equal and increasing manner;
[0036] S102: tableting, the mixture obtained by mixing is directly powder-pressed into tablets.
[0037] Step S101 specifically includes:
[0038] A. In parts by weight, mix 2.5 to 10 parts of amlodipine besylate, 5 to 20 parts of lisinopril and 2 to 10 parts of disintegrant to obtain the mixture ①;
[0039] B. Mix 7.5-30 parts of the filler with the mixture ① of the step A to obtain the mixture ②;
[0040] C. Mix 15-60 parts of filler with the mixture ② of step B to obtain the mixture ③;
[0041] D. Mix the remaining 27.5-110 parts of filler and 0.5-2 parts of lubricant with the mixture ③ in step C to obtain the mixture ④.
[0042] In the preparation method, the mixing time i...
Embodiment 1
[0048] Embodiment 1 amlodipine besylate lisinopril tablet prescription: (in 1000 tablets, unit: g)
[0049]
[0050] Preparation Process:
[0051] (1) mix:
[0052] A, 5g amlodipine besylate, 10g lisinopril and 4g polacrilin potassium were mixed for 5min;
[0053] B, 27g microcrystalline cellulose is mixed with the mixture that step A obtains for 5min;
[0054]C, 54g microcrystalline cellulose is mixed with the mixture that step B obtains for 5min;
[0055] D, the remaining 99g microcrystalline cellulose and 1g sodium fumarate were mixed with the mixture obtained in step C for 15min;
[0056] (2) Tablet compression: the mixture obtained in step D is compressed into tablets, and the tablet hardness is 8kgf.
Embodiment 2
[0057] Example 2 Amlodipine besylate lisinopril tablet prescription: (in 1000 tablets, unit: g)
[0058]
[0059] Preparation Process:
[0060] (1) mix:
[0061] A, 5g amlodipine besylate, 5g lisinopril and 2.5g sodium carboxymethyl starch were mixed for 5min;
[0062] B, 13g pregelatinized starch is mixed with the mixture that step A obtains for 5min;
[0063] C, 26g pregelatinized starch is mixed with the mixture that step B obtains for 5min;
[0064] D, remaining 48g pregelatinized starch and 1g magnesium stearate were mixed with the mixture obtained in step C for 15min;
[0065] (2) Tablet compression: the mixture obtained in step D is compressed into tablets, and the tablet hardness is 9kgf.
[0066] The application effect of the present invention will be described in detail below in conjunction with detection.
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com