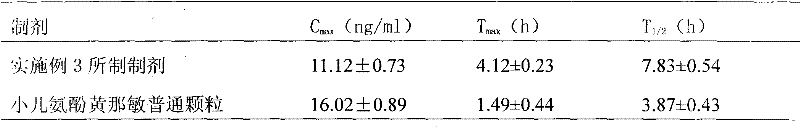Drug composition for treating children upper respiratory tract infection, and preparation method thereof
A technology of upper respiratory tract and composition, applied in the field of pharmaceutical composition and its preparation, which can solve the problems of short action time, profuse sweating, injury to gastric mucosa, etc., achieve rapid onset of action, prolong drug action time, and relieve colds The effect of symptoms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 tablet
[0032] Prepare 1000 tablets
[0033] Component Immediate Release Mixture Extended Release Mixture
[0034] Acetaminophen 50.0g 75.0g
[0035] Chlorpheniramine maleate 0.2g 0.3g
[0036] Artificial Bezoar 2.0g 3.0g
[0037] Microcrystalline Cellulose 12.5g
[0038] Pregelatinized starch 12.5g
[0039] Appropriate amount of starch slurry (10%)
[0040] Hypromellose 50.0g
[0041] PVP solution (5%) appropriate amount
[0042] Magnesium stearate 0.5g 0.7g
[0043] Preparation Process:
[0044] Immediate release part: acetaminophen, chlorpheniramine maleate, artificial bezoar mixed with microcrystalline cellulose and pregelatinized starch, added 10% (g / v) starch slurry to granulate, dried, granulated, and Magnesium stearate mixed well.
[0045] Sustained-release part: acetaminophen, chlorpheniramine maleate, artificial bezoar and hypromellose are mixed evenly, added 5% PVP (g / v) solution to granulate, dried, granulated, and stearic acid The mag...
Embodiment 2
[0046] Embodiment 2 capsules
[0047] Prepare 1000 capsules
[0048] Component Immediate release part Sustained release part
[0049] Acetaminophen 62.5g 62.5g
[0050] Chlorpheniramine Maleate 0.25g 0.25g
[0051] Artificial Bezoar 2.5g 2.5g
[0052] Microcrystalline Cellulose 16.5g
[0053] Pregelatinized starch 16.5g
[0054] Appropriate amount of starch slurry (10%)
[0055] Hypromellose 30.0g
[0056] PVP solution (5%) appropriate amount
[0057] Magnesium stearate 0.6g
[0058] Copolymerized methacrylic acid 6.5g
[0059] Appropriate amount of talcum powder
[0060] Triethyl citrate 1.2g
[0061] Appropriate amount of pure water
[0062] Preparation Process:
[0063] Immediate-release part: acetaminophen, chlorpheniramine maleate, artificial bezoar, mixed with microcrystalline cellulose and pregelatinized starch, added with starch slurry to granulate, dried, granulated, and evenly mixed with magnesium stearate.
[0064] Sustained-release part: acetaminophen...
Embodiment 3
[0065]Embodiment 3 granules prepare 1000 bags
[0066] Component Immediate Release Mixture Extended Release Mixture
[0067] Acetaminophen 50.0g 75.0g
[0068] Chlorpheniramine maleate 0.2g 0.3g
[0069] Artificial Bezoar 2.0g 3.0g
[0070] Hypromellose (E5) 1.5g
[0071] Microcrystalline Cellulose 10.0g
[0072] Hypromellose (K4M) 40.0g
[0073] PVP solution (5%) appropriate amount
[0074] Sucrose Appropriate amount Appropriate amount
[0075] Preparation Process:
[0076] Mix acetaminophen, chlorpheniramine maleate, artificial bezoar, microcrystalline cellulose and hypromellose (K4M) in a slow-release ratio evenly, add PVP solution to granulate, dry, granulate, and use for granules The other part is coated with a suspension made of paracetamol, chlorpheniramine maleate, artificial bezoar and hypromellose (E5), mixed evenly with sucrose, bagged, and obtained.
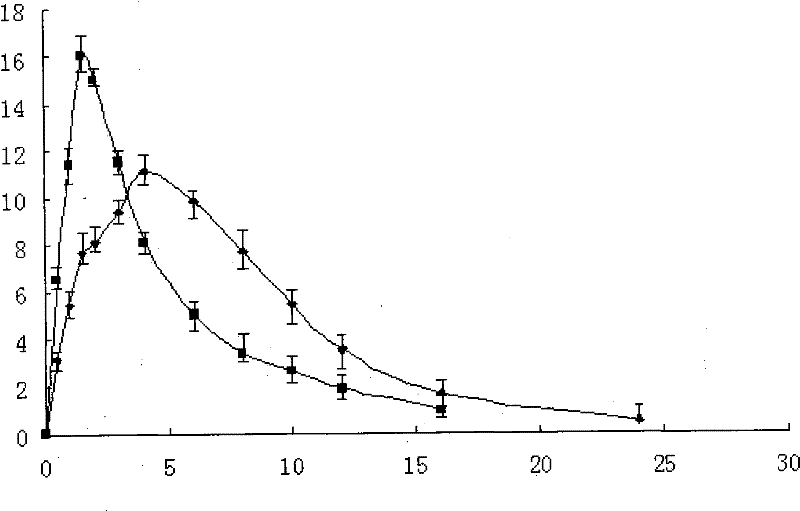
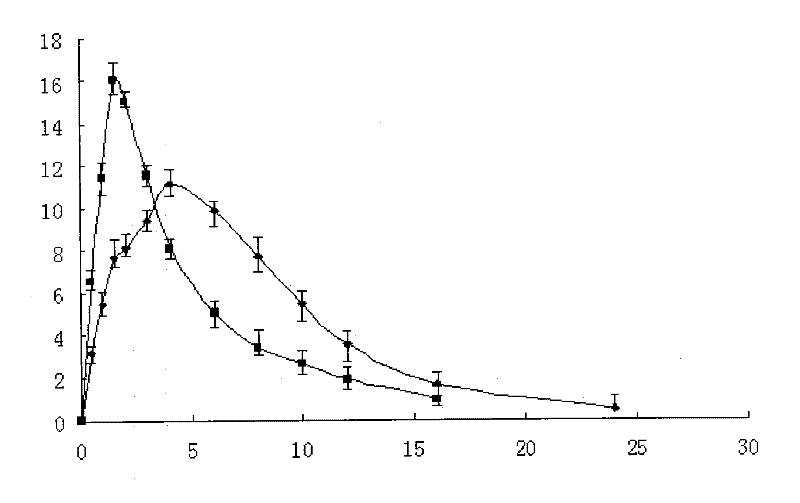
[0077] Preparations prepared in the above-mentioned examples and common preparations in vitro release curv...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com