hpma-docetaxel or gemcitabine conjugates and uses thereof
一种吉西他滨、多西他赛的技术,应用在HPMA-多西他赛或吉西他滨缀合物及其用途领域,能够解决肿瘤转移性疾病等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0100] Example 1: Synthesis of HPMA-gemcitabine or docetaxel conjugates for drug delivery
[0101] HPMA comonomer drug conjugates were synthesized by polymerization of HPMA monomer and activated MA-GFLG-drug (SEQ ID NO: 1 ) comonomer in different molar ratios.
[0102] 1) Synthesis of HPMA comonomer
[0103] The synthesis of HPMA monomer was carried out as previously described (Kopecek and Bazilova, Eur. Polym. J., 9: 7-14 (1973)), as figure 1 shown. The MA-GG-ONp comonomer was prepared by a modified multistep reaction (Kopecek et al., Ann. N Y Acad. Sci., 446:93-104 (1985)).
[0104] plan 1
[0105]
[0106] To a solution of 1-amino-2propanol (65.6ml, 0.84mol) in 250ml of acetonitrile was added dropwise freshly distilled methacryloyl chloride (MACl) (41ml, 0.42mol, in 20ml of acetonitrile) at -5°C with vigorous stirring . A small amount of the inhibitor tert-octylcatechol was added to the solution. The reaction mixture was stirred at room temperature for an additional ...
Embodiment 2
[0148] Embodiment 2: biological test
[0149] 1) Growth of cancer cell lines
[0150] Cancer cell lines used to assay the effects of HPMA-drug conjugates were obtained from the following sources: human MDA-MB-231 (breast), HCT116 (colon) and PANC-1 (pancreas) from the American Standard Culture Center (ATCC) ( Manassas, VA). UMRC2 (kidney) was from the National Cancer Institute (Bethesda, MD). Cells were maintained in Dulbecco's Modified Eagle's Medium ("DMEM", Invitrogen) supplemented with 10% FBS, P / S and 10 mM HEPES. All cells were incubated at 37°C in humidified 5% CO 2 incubate.
[0151] 2) In vitro cell proliferation assay for human tumor cell lines
[0152] Growth inhibition assays of HPMA-drug conjugates against human cancer cell lines were performed using the sulforhodamine B ("SRB") method (Skehan et al., J. National Cancer Institute, 82: 1107-1112 (1990)). Briefly, exponentially growing cancer cells were treated with 2-3x10 3 Cells / well were seeded in 96-well ...
Embodiment 3
[0159] Example 3: Xenotransplantation Studies
[0160] To observe tumor growth inhibition in animal models, HPMA-conjugated docetaxel or gemcitabine was used on nude mouse xenograft models as described below.
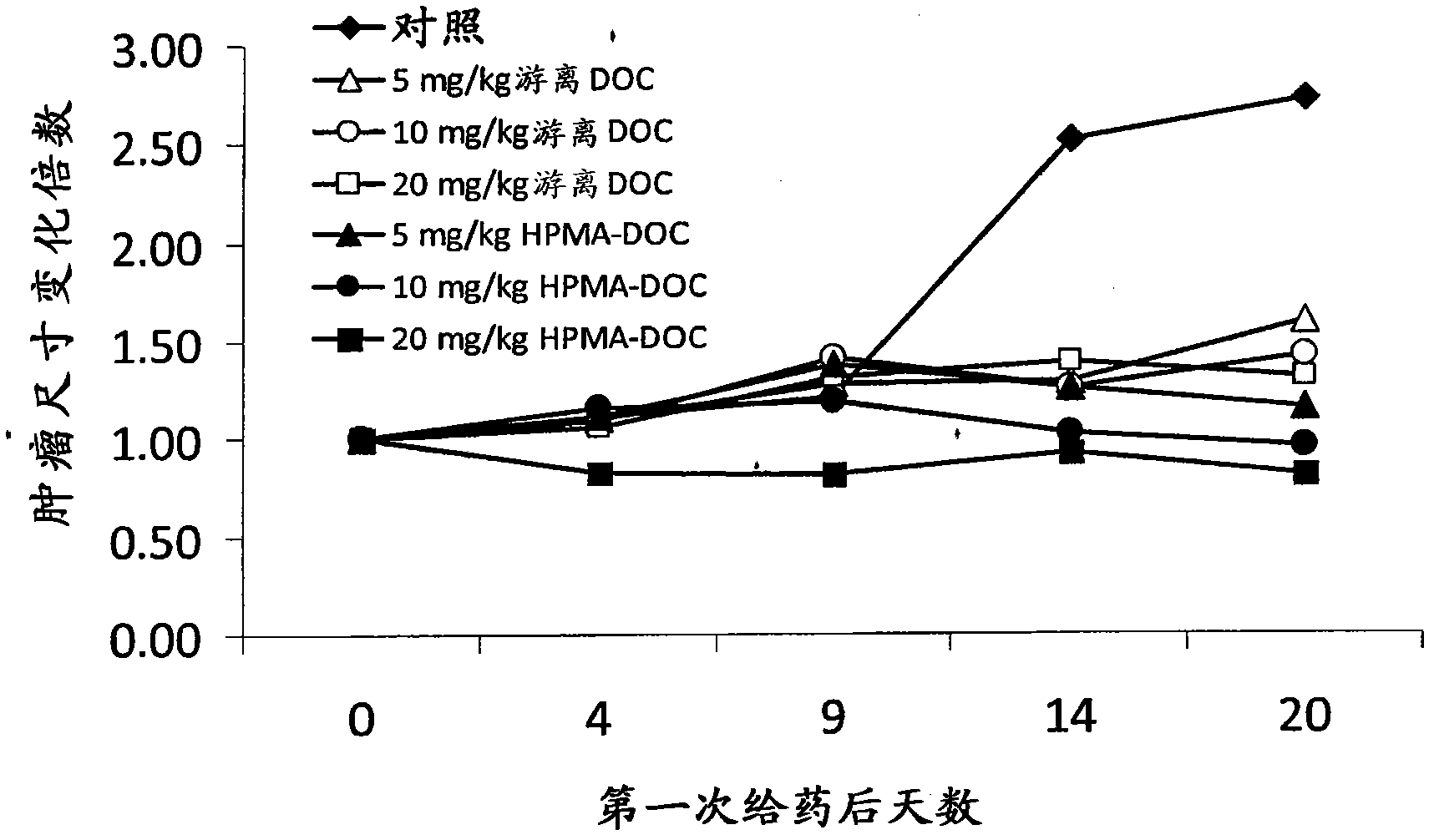
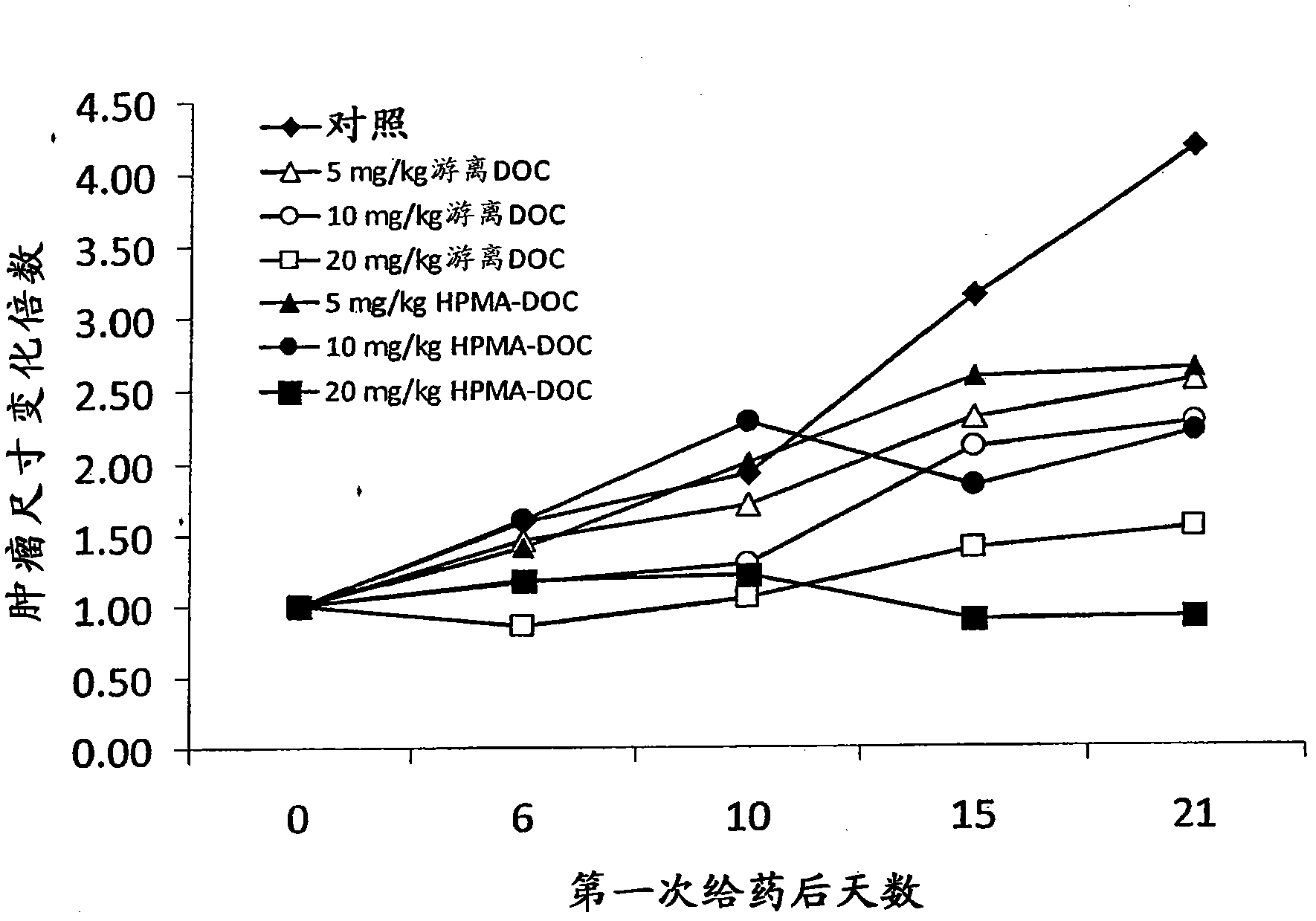
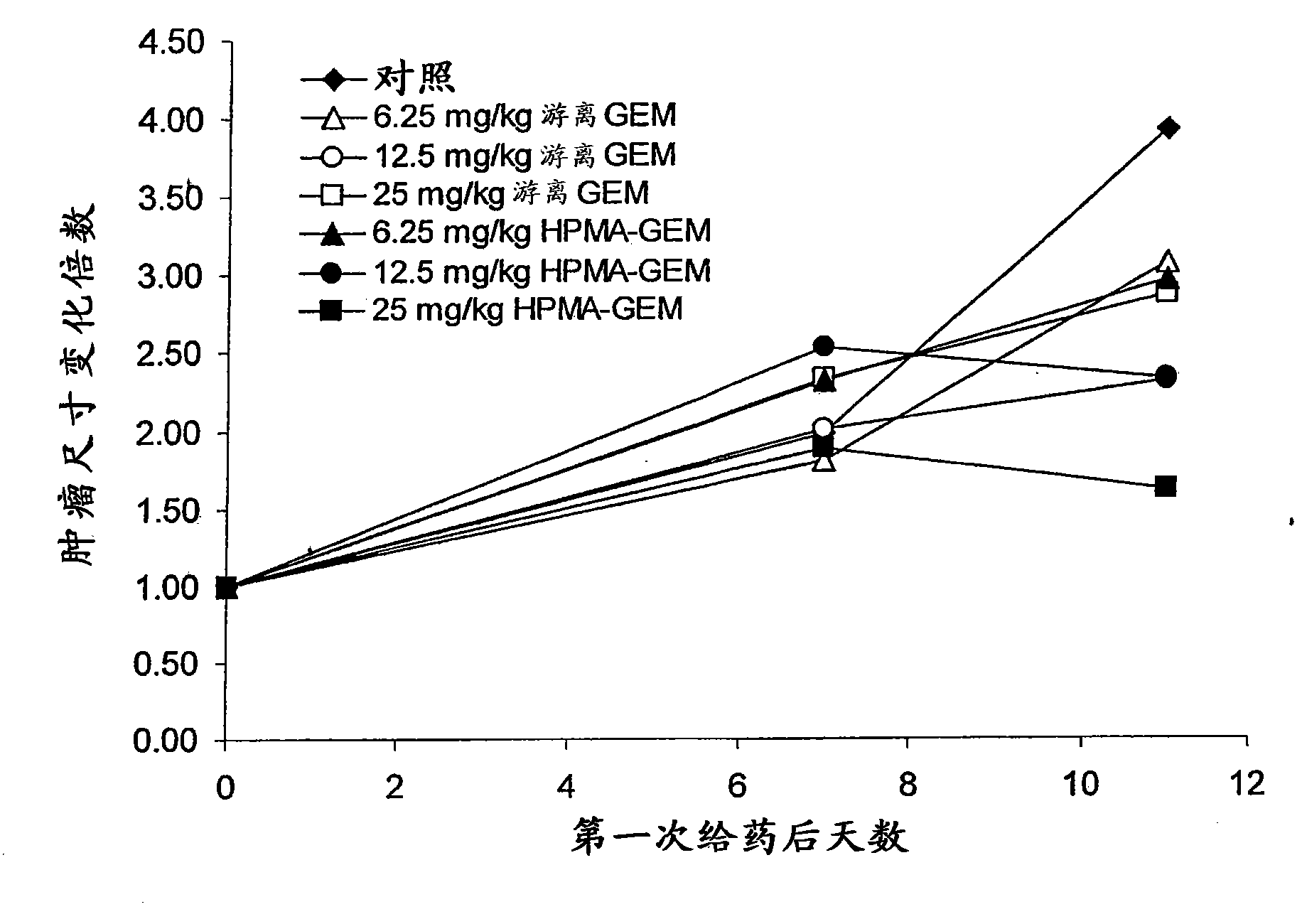
[0161] Mia-Paca human pancreatic cancer cell or HCT116 cell suspension (3x10 6 cells) were subcutaneously injected into the lower flank of six-week-old female FoxN1 nude mice. When the tumor reaches an appropriate size (eg, about 50-60mm 3 volume), the mice were injected intravenously with phosphate-buffered saline (PBS) alone or the drug conjugate of the invention every 4-5 days. Animals are monitored for several weeks until control tumors reach, eg, about 1 cm in diameter, at which point animals are euthanized. Tumor size and tumor composition were determined and reported as fold change in tumor size ( Figure 1-Figure 3 ).
[0162] Using the model described in this example, it was shown that drug conjugates of the invention, such as those described in Example 1,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com