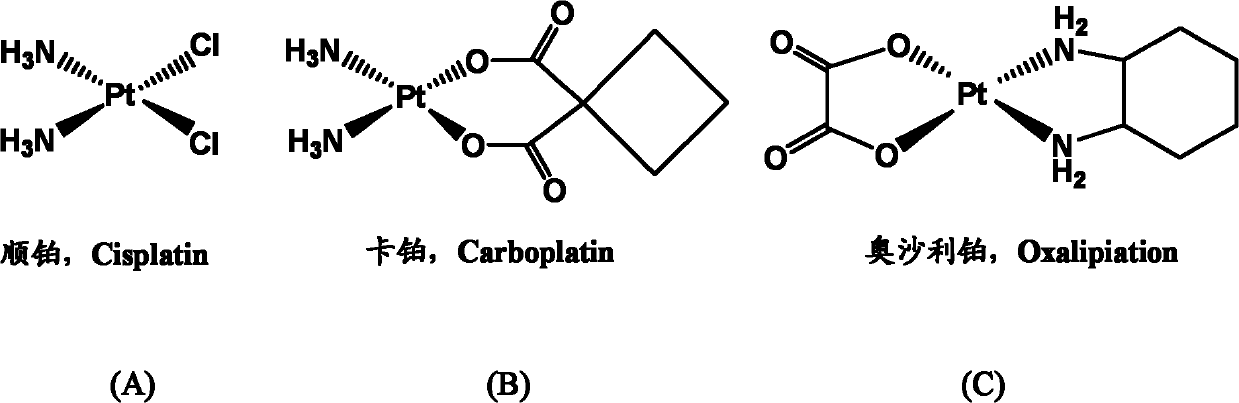
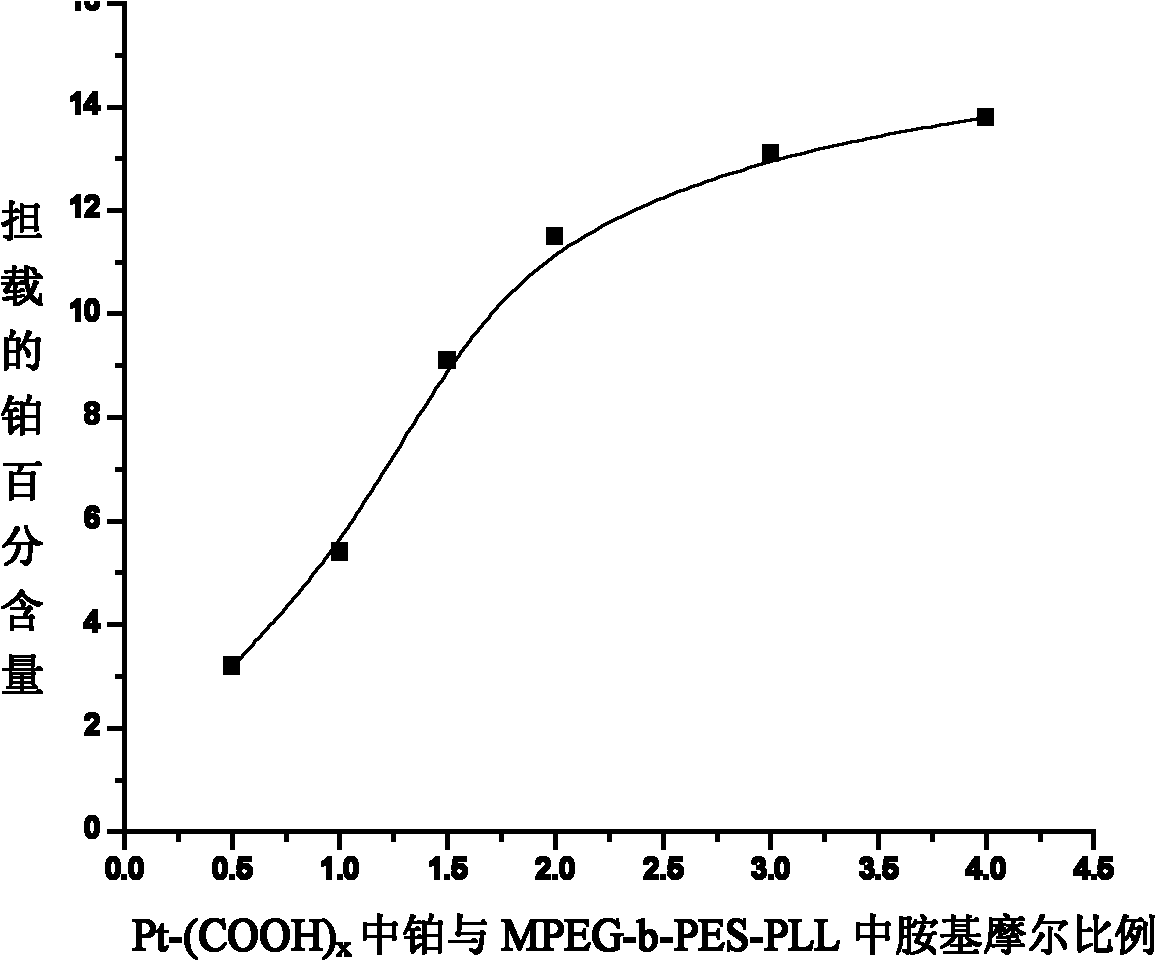
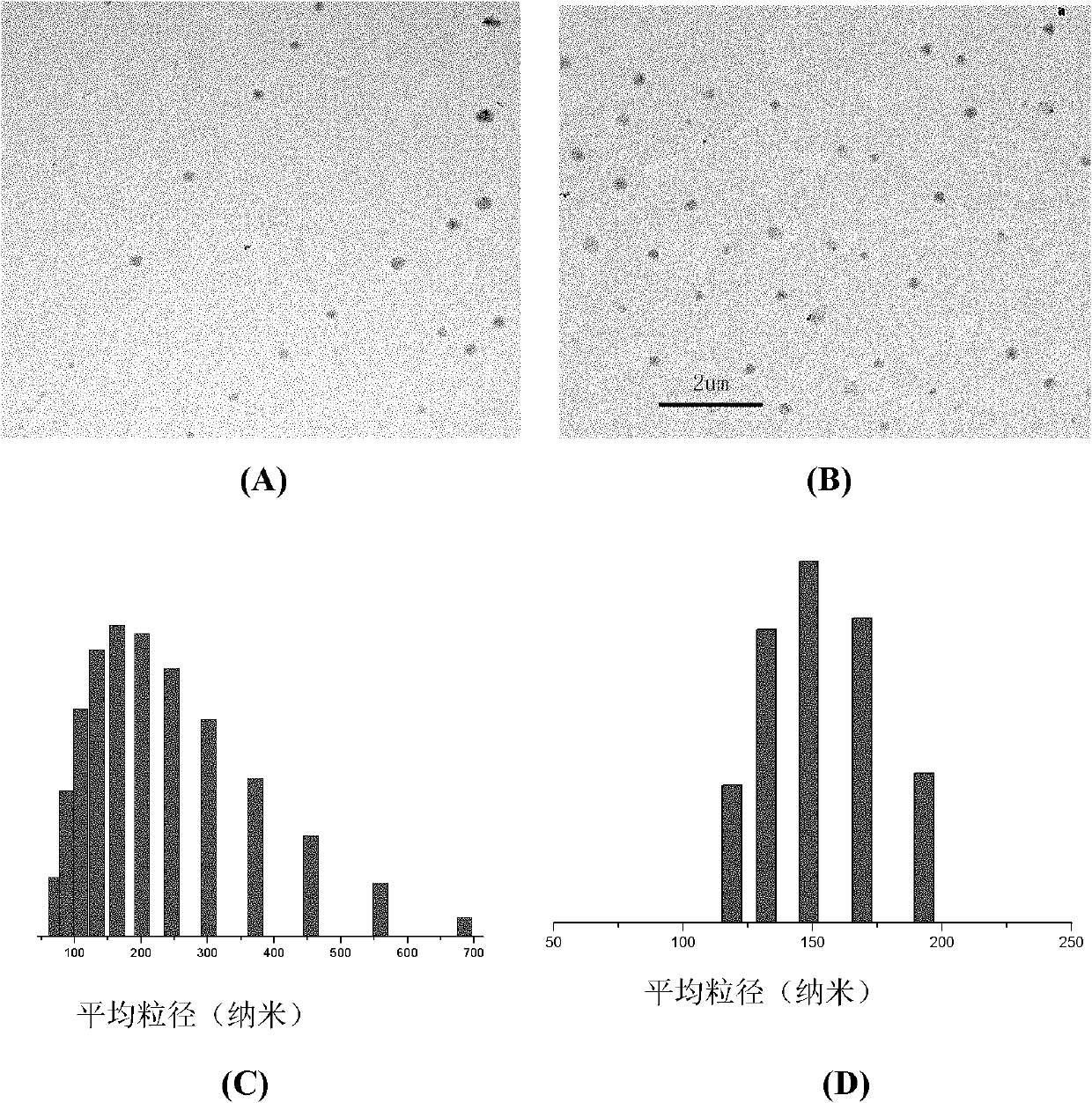
Nano micelle of biodegradable macromolecular-bonding Pt(IV) anti-cancer medicament and preparation method thereof
A technology of biodegradable and anticancer drugs, which is applied in the direction of antineoplastic drugs, drug combinations, and pharmaceutical formulations, and can solve problems such as failure to meet the use requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] Embodiment 1: the preparation of polyethylene glycol-b-polyester block polymer:
[0084] With ε-caprolactone as the cyclic ester monomer, 10 g of polyethylene glycol monomethyl ether with a molecular weight of 5000 was selected as the initiator, dissolved in 50 mL of toluene, and subjected to azeotropic distillation for 1 hour to remove residual water. Add 5gε-caprolactone, then add 100μL of 10% (w / w) stannous octoate toluene solution, stir evenly, heat to 120°C, polymerize for 12 hours and cool to room temperature, dissolve the polymer with 50mL chloroform, and then use 500mL Precipitate the polymer with a methanol / ether mixture with a volume ratio of 1:2; dissolve the precipitated polymer with 50 mL of chloroform, and then use 500 mL of a methanol / ether mixture with a volume ratio of 1:2 for precipitation; the product was vacuum-dried at room temperature for 12 hours. Calculate the polymerization yield by weighing. use 1 The HNMR method determines the molecular weig...
Embodiment 2
[0088] Example 2: Azidation of terminal hydroxyl groups of polyethylene glycol-b-polyester
[0089] Add 10g (1.4mmol) EG to a 500mL reaction flask 5000 -b-PCL 2280 And 0.4mL triethylamine, dissolved with 100mL chloroform under stirring. The reaction flask was cooled in an ice bath at 0°C, and 3.6 mL of 10% (w / v) sulfonyl chloride (CH 3 SO 2 Cl) in chloroform solution, after the reaction was carried out for 4 hours, the reaction product was poured into 500 mL of methanol / ether mixture (volume ratio 1:2) to precipitate a white solid.
[0090] Dissolve the precipitate in 100 mL of N,N-dimethylformamide (DMF), add 1.0 g NaN 3 , stirred at room temperature for 3 days. The solvent was then removed in vacuo, filtered, precipitated, and dried in vacuo to obtain the azidation product MPEG 5000 -b-PCL 2280 -N 3 .
[0091] The kind of polymer and methanesulfonyl chloride, triethylamine consumption, the consumption of sodium azide and the reaction time are listed in Table 3, and ...
Embodiment 3
[0094] Embodiment 3: the terminal azido group of polyethylene glycol-b-polyester is reduced to the terminal amino group, prepares MPEG-b-PES-NH 2 Methods
[0095] MPEG 5000 -b-PCL 2280 -N 3 As an example, the specific operation of the catalytic hydrogenation reaction is as follows: suspend 4g of polyethylene glycol-b-polyester with terminal azidation in a mixed solvent of 50mL of methanol and 300mL of tetrahydrofuran, transfer it to a hydrogenation autoclave, and add Pd(OH) supported on activated carbon 2 Palladium carbon catalyst Pd(OH) with a mass content of 10% 2 / C 600mg, feed hydrogen to make the hydrogen pressure reach 2.5MPa, maintain this pressure and keep constant temperature 50°C, and react for 24 hours. Release the material after pressure release, remove the catalyst by filtration, remove the solvent by evaporation, dissolve with 100mL chloroform, and precipitate 500mL methanol / ether (volume ratio 1:2) mixed solution to obtain MPEG-b-PCL-NH 2 White product, yi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com